Anaplasma phagocytophilum reduces neutrophil apoptosis in vivo
- PMID: 12654818
- PMCID: PMC152089
- DOI: 10.1128/IAI.71.4.1995-2001.2003
Anaplasma phagocytophilum reduces neutrophil apoptosis in vivo
Abstract
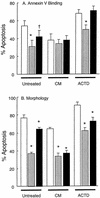
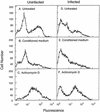
Ovine neutrophils spontaneously underwent apoptosis during culture in vitro, as assessed by morphological changes and exposure of annexin V binding sites on their cell surfaces. The addition of conditioned medium from concanavalin A-treated ovine peripheral blood mononuclear cells (PBMC) could partially protect against this progression into apoptosis, but dexamethasone and sodium butyrate could not. Actinomycin D accelerated the rate at which ovine neutrophils underwent apoptosis. Neutrophils isolated from sheep experimentally infected with Anaplasma phagocytophilum showed significantly delayed apoptosis during culture ex vivo, and the addition of conditioned medium from PBMC to these cells could not delay apoptosis above the protective effects observed after in vivo infection. The ability of neutrophils from A. phagocytophilum-infected sheep to activate a respiratory burst was increased compared to the activity measured in neutrophils from uninfected sheep, but chemotaxis was decreased in neutrophils from infected sheep. These data are the first demonstration that in vivo infection with A. phagocytophilum results in changes in rates of apoptosis of infected immune cells. This may help explain how these bacteria replicate in these normally short-lived cells.
Figures



References
-
- Aga, E., D. M. Katschinski, G. Van Zandbergen, H. Laufs, B. Hansen, K. Muller, W. Solbach, and T. Laskay. 2002. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169:898-905. - PubMed
-
- Akgul, C., D. A. Moulding, and S. W. Edwards. 2001. Molecular control of neutrophil apoptosis. FEBS Lett. 487:318-322. - PubMed
-
- Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper midwest United States: a new emerging species? JAMA 272:212-218. - PubMed
-
- Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by downregulating gp91phox1. J. Immunol. 164:3946-3949. - PubMed
-
- Bedner, E., P. Burfeind, T.-C Hseih, J. M. Wu, M. E. Aguero-Rosenfeld, M. R. Melamed, H. W. Horowitz, G. P. Wormser, and Z. Darzynkiewicz. 1998. Cell cycle effects and induction of apoptosis caused by infection of HL-60 cells with human granulocytic ehrlichiosis pathogen measured by flow and laser scanning cytometry. Cytometry 33:47-55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

