Macula densa cell signaling involves ATP release through a maxi anion channel
- PMID: 12655045
- PMCID: PMC153091
- DOI: 10.1073/pnas.0736323100
Macula densa cell signaling involves ATP release through a maxi anion channel
Abstract
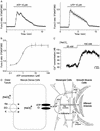
Macula densa cells are unique renal biosensor cells that detect changes in luminal NaCl concentration ([NaCl](L)) and transmit signals to the mesangial cellafferent arteriolar complex. They are the critical link between renal salt and water excretion and glomerular hemodynamics, thus playing a key role in regulation of body fluid volume. Since identification of these cells in the early 1900s, the nature of the signaling process from macula densa cells to the glomerular contractile elements has remained unknown. In patch-clamp studies of macula densa cells, we identified an [NaCl](L)-sensitive ATP-permeable large-conductance (380 pS) anion channel. Also, we directly demonstrated the release of ATP (up to 10 microM) at the basolateral membrane of macula densa cells, in a manner dependent on [NaCl](L), by using an ATP bioassay technique. Furthermore, we found that glomerular mesangial cells respond with elevations in cytosolic Ca(2+) concentration to extracellular application of ATP (EC(50) 0.8 microM). Importantly, we also found increases in cytosolic Ca(2+) concentration with elevations in [NaCl](L), when fura-2-loaded mesangial cells were placed close to the basolateral membrane of macula densa cells. Thus, cell-to-cell communication between macula densa cells and mesangial cells, which express P2Y(2) receptors, involves the release of ATP from macula densa cells via maxi anion channels at the basolateral membrane. This mechanism may represent a new paradigm in cell-to-cell signal transduction mediated by ATP.
Figures



References
-
- Bell P D, Lapointe J-Y. Clin Exp Pharmacol Physiol. 1997;25:541–547. - PubMed
-
- Schnermann J. Am J Physiol. 1998;274:R263–R279. - PubMed
-
- Navar L G, Inscho E W, Majid S A, Imig J D, Harrison-Bernard L M, Mitchell K D. Physiol Rev. 1996;76:425–536. - PubMed
-
- Barajas L. Fed Proc. 1981;40:78–86. - PubMed
-
- Yang T, Park J M, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs J P, Schnermann J. J Biol Chem. 2000;275:37922–37929. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

