New clues for platinum antitumor chemistry: kinetically controlled metal binding to DNA
- PMID: 12655051
- PMCID: PMC152970
- DOI: 10.1073/pnas.0737293100
New clues for platinum antitumor chemistry: kinetically controlled metal binding to DNA
Abstract
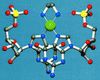
From the metal ions and metal compounds that are known to bind to DNA, many anticancer Pt(II) and Ru(II)Ru(III) compounds are known to have ligand-exchange kinetics in the same order of magnitude as the division of tumor cells. The present article discusses this process in detail with special attention to cisplatin and related compounds and the cellular binding sites and processes of such compounds. Detailed platinated DNA structures are presented and discussed in light of the mechanistic studies of metal antitumor compounds. It is now known that platinum antitumor drugs eventually end up on the DNA. However, it remains a challenge to understand how (fast) they reach the DNA and how they are removed. The kinetics of ligand exchange around platinum appear to play a crucial role, and the possible role of other ligands as intermediates, especially those with S-donor sites, is of great interest. New types of Pt compounds with additional functionalities influencing DNA binding and kinetics are discussed in the context of steric and H-bonding properties. A comparison is made with more sterically crowded Ru complexes. The effects on activity and correlations with structural and kinetic properties are clues in understanding the biological activities of these classes of compounds.
Figures
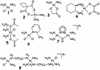



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

