The bioinorganic chemistry of iron in oxygenases and supramolecular assemblies
- PMID: 12655056
- PMCID: PMC152962
- DOI: 10.1073/pnas.0830019100
The bioinorganic chemistry of iron in oxygenases and supramolecular assemblies
Abstract
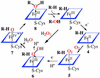
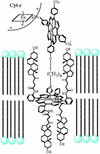
The bioinorganic chemistry of iron is central to life processes. Organisms must recruit iron from their environment, control iron storage and trafficking within cells, assemble the complex, iron-containing redox cofactors of metalloproteins, and manage a myriad of biochemical transformations by those enzymes. The coordination chemistry and the variable oxidation states of iron provide the essential mechanistic machinery of this metabolism. Our current understanding of several aspects of the chemistry of iron in biology are discussed with an emphasis on the oxygen activation and transfer reactions mediated by heme and nonheme iron proteins and the interactions of amphiphilic iron siderophores with lipid membranes.
Figures
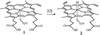




References
-
- Hayaishi O, Katagiri M, Rothberg S. J Am Chem Soc. 1955;77:5450–5451.
-
- Tchen T T, Block K. J Am Chem Soc. 1956;78:1516–1517.
-
- Ortiz de Montellano P R, De Voss J J. Nat Prod Rep. 2002;19:477–493. - PubMed
-
- Sundaramoorty M, Terner J, Poulos T L. Structure (London) 1995;3:1367–1377. - PubMed
-
- Manoj K M, Hager L P. Biochim Biophys Acta. 2001;1547:408–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

