Prostaglandin F2alpha- and FAS-activating antibody-induced regression of the corpus luteum involves caspase-8 and is defective in caspase-3 deficient mice
- PMID: 12657159
- PMCID: PMC152637
- DOI: 10.1186/1477-7827-1-15
Prostaglandin F2alpha- and FAS-activating antibody-induced regression of the corpus luteum involves caspase-8 and is defective in caspase-3 deficient mice
Abstract
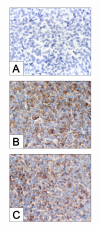
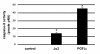
We recently demonstrated that caspase-3 is important for apoptosis during spontaneous involution of the corpus luteum (CL). These studies tested if prostaglandin F2alpha (PGF2alpha) or FAS regulated luteal regression, utilize a caspase-3 dependent pathway to execute luteal cell apoptosis, and if the two receptors work via independent or potentially shared intracellular signaling components/pathways to activate caspase-3. Wild-type (WT) or caspase-3 deficient female mice, 25-26 days old, were given 10 IU equine chorionic gonadotropin (eCG) intraperitoneally (IP) followed by 10 IU human chorionic gonadotropin (hCG) IP 46 h later to synchronize ovulation. The animals were then injected with IgG (2 micrograms, i.v.), the FAS-activating antibody Jo2 (2 micrograms, i.v.), or PGF2alpha (10 micrograms, i.p.) at 24 or 48 h post-ovulation. Ovaries from each group were collected 8 h later for assessment of active caspase-3 enzyme and apoptosis (measured by the TUNEL assay) in the CL. Regardless of genotype or treatment, CL in ovaries collected from mice injected 24 h after ovulation showed no evidence of active caspase-3 or apoptosis. However, PGF2alpha or Jo2 at 48 h post-ovulation and collected 8 h later induced caspase-3 activation in 13.2 +/- 1.8% and 13.7 +/- 2.2 % of the cells, respectively and resulted in 16.35 +/- 0.7% (PGF2alpha) and 14.3 PlusMinus; 2.5% TUNEL-positive cells when compared to 1.48 +/- 0.8% of cells CL in IgG treated controls. In contrast, CL in ovaries collected from caspase-3 deficient mice whether treated with PGF2alpha, Jo2, or control IgG at 48 h post-ovulation showed little evidence of active caspase-3 or apoptosis. CL of WT mice treated with Jo2 at 48 h post-ovulation had an 8-fold increase in the activity of caspase-8, an activator of caspase-3 that is coupled to the FAS death receptor. Somewhat unexpectedly, however, treatment of WT mice with PGF2alpha at 48 h post-ovulation resulted in a 22-fold increase in caspase-8 activity in the CL, despite the fact that the receptor for PGF2alpha has not been shown to be directly coupled to caspase-8 recruitment and activation. We hypothesize that PGF2alpha initiates luteolysis in vivo, at least in part, by increasing the bioactivity or bioavailability of cytokines, such as FasL and that multiple endocrine factors work in concert to activate caspase-3-driven apoptosis during luteolysis.
Figures


Similar articles
-
Caspase-3 is a pivotal mediator of apoptosis during regression of the ovarian corpus luteum.Endocrinology. 2002 Apr;143(4):1495-501. doi: 10.1210/endo.143.4.8726. Endocrinology. 2002. PMID: 11897708
-
Peroxiredoxin 2 regulates PGF2α-induced corpus luteum regression in mice by inhibiting ROS-dependent JNK activation.Free Radic Biol Med. 2017 Jul;108:44-55. doi: 10.1016/j.freeradbiomed.2017.03.013. Epub 2017 Mar 18. Free Radic Biol Med. 2017. PMID: 28323129
-
Effects of prostaglandin F2α (PGF2α) on cell-death pathways in the bovine corpus luteum (CL).BMC Vet Res. 2019 Nov 21;15(1):416. doi: 10.1186/s12917-019-2167-3. BMC Vet Res. 2019. PMID: 31752870 Free PMC article.
-
Inter- and intra-cellular mechanisms of prostaglandin F2alpha action during corpus luteum regression in cattle.Soc Reprod Fertil Suppl. 2010;67:305-24. doi: 10.7313/upo9781907284991.025. Soc Reprod Fertil Suppl. 2010. PMID: 21755681 Review.
-
Blood flow: a key regulatory component of corpus luteum function in the cow.Domest Anim Endocrinol. 2005 Aug;29(2):329-39. doi: 10.1016/j.domaniend.2005.03.011. Domest Anim Endocrinol. 2005. PMID: 15888379 Review.
Cited by
-
Long-term apoptosis-related protein expression in the diabetic mouse ovary.PLoS One. 2018 Sep 7;13(9):e0203268. doi: 10.1371/journal.pone.0203268. eCollection 2018. PLoS One. 2018. PMID: 30192809 Free PMC article.
-
Endoplasmic reticulum stress-mediated apoptotic pathway is involved in corpus luteum regression in rats.Reprod Sci. 2015 May;22(5):572-84. doi: 10.1177/1933719114553445. Epub 2014 Oct 20. Reprod Sci. 2015. PMID: 25332219 Free PMC article.
-
Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells.Mol Endocrinol. 2008 Sep;22(9):2128-40. doi: 10.1210/me.2008-0095. Epub 2008 Jul 7. Mol Endocrinol. 2008. PMID: 18606860 Free PMC article.
-
Type-dependent differences in Fas expression and phagocytes distribution in rat corpora lutea during natural regression: an immunohistochemical evidence.J Vet Med Sci. 2017 Jan 10;78(12):1771-1777. doi: 10.1292/jvms.16-0199. Epub 2016 Aug 19. J Vet Med Sci. 2017. PMID: 27546215 Free PMC article.
-
Role of the DLL4-NOTCH system in PGF2alpha-induced luteolysis in the pregnant rat.Biol Reprod. 2011 May;84(5):859-65. doi: 10.1095/biolreprod.110.088708. Epub 2011 Jan 5. Biol Reprod. 2011. PMID: 21209419 Free PMC article.
References
-
- Niswender GD. Molecular control of luteal secretion of progesterone. Reproduction. 2002;123:333–339. - PubMed
-
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80:1–29. - PubMed
-
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev. 1999;79:263–323. - PubMed
-
- Pate JL, Landis Keyes P. Immune cells in the corpus luteum: friends or foes? Reproduction. 2001;122:665–676. - PubMed
-
- Quirk SM, Harman RM, Huber SC, Cowan RG. Responsiveness of mouse corpora luteal cells to Fas antigen (CD95)-mediated apoptosis. Biol Reprod. 2000;63:49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

