The binding of 2-(4'-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component
- PMID: 12657667
- PMCID: PMC6741999
- DOI: 10.1523/JNEUROSCI.23-06-02086.2003
The binding of 2-(4'-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component
Abstract
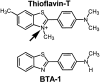
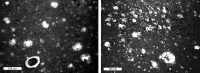
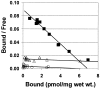
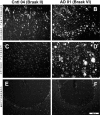
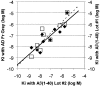
2-(4'-methylaminophenyl)benzothiazole (BTA-1) is an uncharged derivative of thioflavin-T that has high affinity for Abeta fibrils and shows very good brain entry and clearance. In this study, we asked whether BTA-1, at concentrations typical of those achieved during positron emission tomography (PET) studies, could specifically bind to amyloid deposits in the complex milieu of human brain or whether amyloid binding was overshadowed by nonspecific binding, found even in brains that did not contain amyloid deposits. We quantitatively assessed [3H]BTA-1 binding to crude homogenates of postmortem brain obtained from nine Alzheimer's disease (AD) subjects, eight controls, and six subjects with non-AD dementia. BTA-1 binding was >10-fold higher in AD brain, and the majority (94%) of the binding was specific (displaceable). High-affinity [3H]BTA-1 was observed only in AD brain gray matter and was not present in control brain gray matter, AD brain white matter, or cerebellum. The K(d) of [3H]BTA-1 for binding to AD brain (5.8 +/- 0.90 nm) was very similar to the K(d) for binding to synthetic Abeta fibrils. In addition, the K(i) of various BTA analogs for inhibition of [3H]BTA-1 binding to AD brain homogenates was very similar to their K(i) for inhibition of [3H]BTA-1 binding to synthetic Abeta fibrils. Nanomolar concentrations of [3H]BTA-1 did not appear to bind to neurofibrillary tangles. Finally, BTA-1 did not appear to bind significantly to common neuroreceptors or transporter sites. These data suggest that the binding of BTA-1 to AD brain is dominated by a specific interaction with Abeta amyloid deposits.
Figures

References
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Schenk D, Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
-
- Bennett JP, Yamamura HI. Neurotransmitter, hormone, or drug receptor binding methods. In: Yamamura HI, Enna SJ, Kuhar MJ, editors. Neurotransmitter receptor binding. Raven; New York: 1985. pp. 61–89.
-
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. - PubMed
-
- Goedert M, Spillantini MG. Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer's disease. Biochim Biophys Acta. 2000;1502:110–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
