Voltage-gated sodium channels and ankyrinG occupy a different postsynaptic domain from acetylcholine receptors from an early stage of neuromuscular junction maturation in rats
- PMID: 12657669
- PMCID: PMC6742006
- DOI: 10.1523/JNEUROSCI.23-06-02102.2003
Voltage-gated sodium channels and ankyrinG occupy a different postsynaptic domain from acetylcholine receptors from an early stage of neuromuscular junction maturation in rats
Abstract
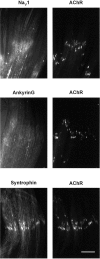
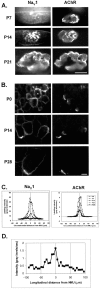
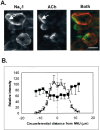
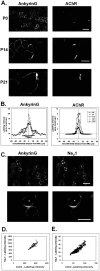
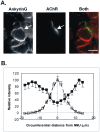
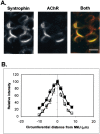
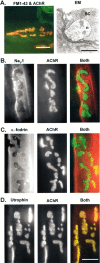
Spatial segregation of membrane proteins is a feature of many excitable cells. In skeletal muscle, clusters of acetylcholine receptors (AChRs) and voltage-gated sodium channels (Na(V)1s) occupy distinct domains at the neuromuscular junction (NMJ). We used quantitative immunolabeling of developing rat soleus muscles to study the mechanism of ion channel segregation and Na(V)1 clustering at NMJs. When Na(V)1s can first be detected, at birth, they already occupy a postsynaptic domain that is distinct from that occupied by AChRs. At this time, Na(V)1s are expressed only in a diffuse area that extends 50-100 microm from the immature NMJ. However, in the region of the high-density AChR cluster at NMJ itself, Na(V)1s are actually present in lower density than in the immediately surrounding membrane. These distinctive features of the Na(V)1 distribution at birth are closely correlated with the distribution of ankyrinG immunolabeling. This suggests that an interaction with ankyrinG plays a role in the initial segregation of Na(V)1s from AChRs. Both Na(V)1 and ankyrinG become clustered at the NMJ itself 1-2 weeks after birth, coincident with the formation of postsynaptic folds. Syntrophin immunolabeling codistributes with AChRs and never resembles that for Na(V)1 or ankyrinG. Therefore, syntrophin is unlikely to play an important part in the initial accumulation of Na(V)1 at the NMJ. These findings suggest that the segregation of Na(V)1 from AChRs begins early in NMJ formation and occurs as a result of the physical exclusion of Na(V)1 and ankyrinG from the region of nerve-muscle contact rather than by a process of active clustering.
Figures

References
-
- Awad SS, Buckel A, Lightowlers RN, Lømo T, Stocksley MA, Wood SJ, Young C, Slater CR. Accumulation of voltage-gated sodium channels at rat neuromuscular junctions. Soc Neurosci Abstr. 1999;25:1021.
-
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. βIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
