Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington's disease
- PMID: 12657678
- PMCID: PMC6742008
- DOI: 10.1523/JNEUROSCI.23-06-02193.2003
Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington's disease
Abstract
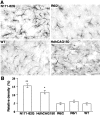
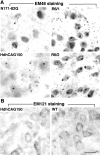
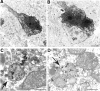
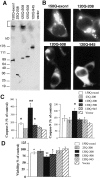
Huntington's disease (HD) mouse models that express N-terminal huntingtin fragments show rapid disease progression and have been used for developing therapeutics. However, light microscopy reveals no significant neurodegeneration in these mice. It remains unclear how mutant huntingtin induces neurodegeneration. Using caspase staining, terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling, and electron microscopy, we observed that N171-82Q mice, which express the first 171 aa of mutant huntingtin, displayed more degenerated neurons than did other HD mouse models. The neurodegeneration was also evidenced by increased immunostaining for glial fibrillary acidic protein and ultrastructural features of apoptosis. R6/2 mice, which express exon 1 of mutant huntingtin, showed dark, nonapoptotic neurons and degenerated mitochondria associated with mutant huntingtin. In HD repeat knock-in mice (HdhCAG150), which express full-length mutant huntingtin, degenerated cytoplasmic organelles were found in both axons and neuronal cell bodies in association with mutant huntingtin that was not labeled by an antibody to huntingtin amino acids 342-456. Transfection of cultured cells with mutant huntingtin revealed that an N-terminal huntingtin fragment (amino acids 1-208 plus a 120 glutamine repeat) caused a greater increase in caspase activity than did exon 1 huntingtin and longer huntingtin fragments. These results suggest that context-dependent neurodegeneration in HD may be mediated by different N-terminal huntingtin fragments. In addition, this study has identified neurodegenerative markers for the evaluation of therapeutic treatments in HD mouse models.
Figures




Similar articles
-
Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington's disease.J Neurosci. 2002 Sep 15;22(18):7862-72. doi: 10.1523/JNEUROSCI.22-18-07862.2002. J Neurosci. 2002. PMID: 12223539 Free PMC article.
-
Full length mutant huntingtin is required for altered Ca2+ signaling and apoptosis of striatal neurons in the YAC mouse model of Huntington's disease.Neurobiol Dis. 2008 Jul;31(1):80-8. doi: 10.1016/j.nbd.2008.03.010. Epub 2008 Apr 16. Neurobiol Dis. 2008. PMID: 18502655 Free PMC article.
-
Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington's disease mice.J Neurosci. 2001 Nov 1;21(21):8473-81. doi: 10.1523/JNEUROSCI.21-21-08473.2001. J Neurosci. 2001. PMID: 11606636 Free PMC article.
-
The selective vulnerability of nerve cells in Huntington's disease.Neuropathol Appl Neurobiol. 2001 Feb;27(1):1-21. doi: 10.1046/j.0305-1846.2001.00299.x. Neuropathol Appl Neurobiol. 2001. PMID: 11298997 Review.
-
Genetic manipulations of mutant huntingtin in mice: new insights into Huntington's disease pathogenesis.FEBS J. 2013 Sep;280(18):4382-94. doi: 10.1111/febs.12418. Epub 2013 Jul 31. FEBS J. 2013. PMID: 23829302 Free PMC article. Review.
Cited by
-
Mouse models of polyglutamine diseases: review and data table. Part I.Mol Neurobiol. 2012 Oct;46(2):393-429. doi: 10.1007/s12035-012-8315-4. Epub 2012 Sep 7. Mol Neurobiol. 2012. PMID: 22956270 Free PMC article. Review.
-
The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer's and Huntington's diseases.J Neurosci. 2015 Feb 11;35(6):2817-29. doi: 10.1523/JNEUROSCI.3516-14.2015. J Neurosci. 2015. PMID: 25673868 Free PMC article.
-
New Avenues for the Treatment of Huntington's Disease.Int J Mol Sci. 2021 Aug 4;22(16):8363. doi: 10.3390/ijms22168363. Int J Mol Sci. 2021. PMID: 34445070 Free PMC article. Review.
-
Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice.J Neurosci. 2008 Jun 11;28(24):6182-95. doi: 10.1523/JNEUROSCI.0857-08.2008. J Neurosci. 2008. PMID: 18550760 Free PMC article.
-
Ferroptosis in central nervous system injuries: molecular mechanisms, diagnostic approaches, and therapeutic strategies.Front Cell Neurosci. 2025 Jul 22;19:1593963. doi: 10.3389/fncel.2025.1593963. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40766185 Free PMC article. Review.
References
-
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF. Creatine increases survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiol Dis. 2001;8:479–491. - PubMed
-
- Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000;23:298–304. - PubMed
-
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. - PubMed
-
- Chan EY, Luthi-Carter R, Strand A, Solano SM, Hanson SA, DeJohn MM, Kooperberg C, Chase KO, DiFiglia M, Young AB, Leavitt BR, Cha JH, Aronin N, Hayden MR, Olson JM. Increased huntingtin protein length reduces the number of polyglutamine-induced gene expression changes in mouse models of Huntington's disease. Hum Mol Genet. 2002;11:1939–1951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
