Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway
- PMID: 12657692
- PMCID: PMC6742036
- DOI: 10.1523/JNEUROSCI.23-06-02333.2003
Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway
Abstract
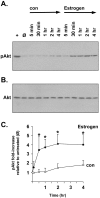
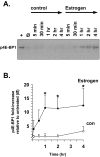
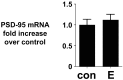
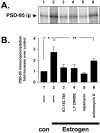
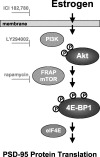
Estrogens induce synaptogenesis in the CA1 region of the dorsal hippocampus during the estrous cycle of the female rat. Functional consequences of such estrogen-mediated synaptogenesis include cyclic changes in neurotransmission and memory. At the molecular level, estrogen stimulates the rapid activation of specific signal transduction pathways, and of particular interest is the activation of Akt (protein kinase B), a key signal transduction intermediate that initiates protein translation by alleviating the downstream translational repression of eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). Using a well established in vitro model system of differentiated NG108-15 neurons to investigate such rapid signaling effects of estrogen, we show that estrogen stimulates the phosphorylation of Akt, an indication of kinase activation, as well as the phosphorylation of 4E-BP1. In turn, the activation of these signaling intermediates suggests a non-genomic mechanism by which estrogen might likewise lead to protein translation of dendrite-localized mRNA transcripts in the hippocampus in vivo. We therefore considered the translation of the dendritic spine scaffolding protein postsynaptic density-95 (PSD-95). Although estrogen does not stimulate a rapid increase in PSD-95 mRNA levels in NG108-15 neurons, we show here that estrogen does however stimulate a rapid increase in PSD-95 new protein synthesis in vitro and that this new protein synthesis is Akt dependent. These results demonstrate an essential role for Akt in estrogen-stimulated dendritic spine protein expression, describe for the first time a signal transduction pathway in PSD-95 expression, and delineate a novel, molecular mechanism by which ovarian hormones might translationally regulate synaptogenesis via activating protein synthesis for dendritic function.
Figures
References
-
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. - PubMed
-
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
