Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord
- PMID: 12657695
- PMCID: PMC6742046
- DOI: 10.1523/JNEUROSCI.23-06-02357.2003
Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord
Abstract
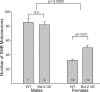
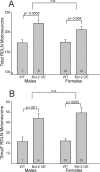
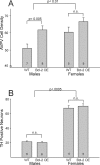
Several sex differences in the nervous system depend on differential cell death during development in males and females. The anti-apoptotic protein, Bcl-2, promotes the survival of many types of neurons during development and in response to injury. To determine whether Bcl-2 might similarly control cell death in sexually dimorphic regions, we compared neuron number in wild-type mice and transgenic mice overexpressing Bcl-2 under the control of a neuron-specific promoter. Three neural areas were examined: the spinal nucleus of the bulbocavernosus (SNB), in which neuron number is greater in males; the retrodorsolateral nucleus (RDLN) of the spinal cord, which exhibits no sex difference in neuron number; and the anteroventral periventricular nucleus (AVPV) of the hypothalamus, in which both overall cell density and the number of tyrosine hydroxylase immunoreactive (TH-ir) neurons are greater in females. Bcl-2 overexpression significantly increased SNB cell number in females, overall cell density of AVPV in males, and RDLN cell number in both sexes. Bcl-2 overexpression did not alter the number of TH-ir neurons in AVPV of males or females. These findings indicate that Bcl-2 can regulate sexually dimorphic cell number in the brain and spinal cord and suggest that Bcl-2 may mediate effects of testosterone on cell survival during neural development. In contrast to the regulation of overall cell density in AVPV, the sex difference in TH cell number apparently is not caused by a Bcl-2-dependent mechanism.
Figures
Similar articles
-
Collapsin response mediator protein 4 affects the number of tyrosine hydroxylase-immunoreactive neurons in the sexually dimorphic nucleus in female mice.Dev Neurobiol. 2013 Jul;73(7):502-17. doi: 10.1002/dneu.22076. Epub 2013 May 14. Dev Neurobiol. 2013. PMID: 23420586
-
Control of cell number in the sexually dimorphic brain and spinal cord.J Neuroendocrinol. 2009 Mar;21(4):393-9. doi: 10.1111/j.1365-2826.2009.01825.x. J Neuroendocrinol. 2009. PMID: 19207822 Free PMC article. Review.
-
Castration reduces motoneuron soma size but not dendritic length in the spinal nucleus of the bulbocavernosus of wild-type and BCL-2 overexpressing mice.J Neurobiol. 2002 Nov 15;53(3):403-12. doi: 10.1002/neu.10103. J Neurobiol. 2002. PMID: 12382267
-
BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice.Endocrinology. 2010 Dec;151(12):5807-17. doi: 10.1210/en.2010-0783. Epub 2010 Oct 6. Endocrinology. 2010. PMID: 20926580 Free PMC article.
-
Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats.J Neuroendocrinol. 2009 Mar;21(4):370-6. doi: 10.1111/j.1365-2826.2009.01855.x. J Neuroendocrinol. 2009. PMID: 19226350 Review.
Cited by
-
Cell death atlas of the postnatal mouse ventral forebrain and hypothalamus: effects of age and sex.J Comp Neurol. 2013 Aug 1;521(11):2551-69. doi: 10.1002/cne.23298. J Comp Neurol. 2013. PMID: 23296992 Free PMC article.
-
Sexually Dimorphic Formation of the Preoptic Area and the Bed Nucleus of the Stria Terminalis by Neuroestrogens.Front Neurosci. 2020 Jul 29;14:797. doi: 10.3389/fnins.2020.00797. eCollection 2020. Front Neurosci. 2020. PMID: 32848568 Free PMC article. Review.
-
Sex differences and organizational effects of androgen in spinal cord motor nuclei.J Undergrad Neurosci Educ. 2003 Fall;2(1):A28-35. Epub 2003 Oct 15. J Undergrad Neurosci Educ. 2003. PMID: 23494208 Free PMC article.
-
A genetic approach to dissect sexually dimorphic behaviors.Horm Behav. 2008 May;53(5):627-37. doi: 10.1016/j.yhbeh.2007.12.012. Epub 2008 Jan 5. Horm Behav. 2008. PMID: 18313055 Free PMC article. Review.
-
Neurons and Glial Cells Are Added to the Female Rat Anteroventral Periventricular Nucleus During Puberty.Endocrinology. 2016 Jun;157(6):2393-402. doi: 10.1210/en.2015-2012. Epub 2016 May 4. Endocrinology. 2016. PMID: 27145006 Free PMC article.
References
-
- Alexander MJ, Kirly ZJ, Leeman SE. Sexually dimorphic distribution of neurotensin/neuromedin N mRNA in the rat preoptic area. J Comp Neurol. 1991;311:84–96. - PubMed
-
- Allsopp TE, Wyatt S, Paterson HF, Davies AM. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993;73:295–307. - PubMed
-
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. - PubMed
-
- Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982;212:118–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
