Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy
- PMID: 12657704
- PMCID: PMC6741996
- DOI: 10.1523/JNEUROSCI.23-06-02440.2003
Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy
Abstract
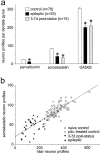
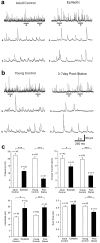
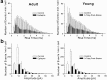
Patients and models of temporal lobe epilepsy have fewer inhibitory interneurons in the dentate gyrus than controls, but it is unclear whether granule cell inhibition is reduced. We report the loss of GABAergic inhibition of granule cells in the temporal dentate gyrus of pilocarpine-induced epileptic rats. In situ hybridization for GAD65 mRNA and immunocytochemistry for parvalbumin and somatostatin confirmed the loss of inhibitory interneurons. In epileptic rats, granule cells had prolonged EPSPs, and they discharged more action potentials than controls. Although the conductances of evoked IPSPs recorded in normal ACSF were not significantly reduced and paired-pulse responses showed enhanced inhibition of granule cells from epileptic rats, more direct measures of granule cell inhibition revealed significant deficiencies. In granule cells from epileptic rats, evoked monosynaptic IPSP conductances were <40% of controls, and the frequency of GABA(A) receptor-mediated spontaneous and miniature IPSCs (mIPSCs) was <50% of controls. Within 3-7 d after pilocarpine-induced status epilepticus, miniature IPSC frequency had decreased, and it remained low, without functional evidence of compensatory synaptogenesis by GABAergic axons in chronically epileptic rats. Both parvalbumin- and somatostatin-immunoreactive interneuron numbers and the frequency of both fast- and slow-rising GABA(A) receptor-mediated mIPSCs were reduced, suggesting that loss of inhibitory synaptic input to granule cells occurred at both proximal/somatic and distal/dendritic sites. Reduced granule cell inhibition in the temporal dentate gyrus preceded the onset of spontaneous recurrent seizures by days to weeks, so it may contribute, but is insufficient, to cause epilepsy.
Figures






Similar articles
-
Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy.J Comp Neurol. 2010 Mar 1;518(5):647-67. doi: 10.1002/cne.22235. J Comp Neurol. 2010. PMID: 20034063 Free PMC article.
-
Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy.J Physiol. 2004 Jun 1;557(Pt 2):473-87. doi: 10.1113/jphysiol.2003.059246. Epub 2004 Mar 19. J Physiol. 2004. PMID: 15034126 Free PMC article.
-
Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy.J Neurosci. 2003 Sep 17;23(24):8471-9. doi: 10.1523/JNEUROSCI.23-24-08471.2003. J Neurosci. 2003. PMID: 13679415 Free PMC article.
-
Status epilepticus in epileptogenesis.Curr Opin Neurol. 1999 Apr;12(2):191-5. doi: 10.1097/00019052-199904000-00010. Curr Opin Neurol. 1999. PMID: 10226752 Review.
-
Temporal Lobe Epileptogenesis: A Focus on Etiology, Neuron Loss, the Latent Period, and Dentate Granule Cell Disinhibition.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 24. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 24. PMID: 39637110 Free Books & Documents. Review.
Cited by
-
Wwox deletion leads to reduced GABA-ergic inhibitory interneuron numbers and activation of microglia and astrocytes in mouse hippocampus.Neurobiol Dis. 2019 Jan;121:163-176. doi: 10.1016/j.nbd.2018.09.026. Epub 2018 Oct 2. Neurobiol Dis. 2019. PMID: 30290271 Free PMC article.
-
Breakdown of spatial coding and interneuron synchronization in epileptic mice.Nat Neurosci. 2020 Feb;23(2):229-238. doi: 10.1038/s41593-019-0559-0. Epub 2020 Jan 6. Nat Neurosci. 2020. PMID: 31907437 Free PMC article.
-
Spatial Distribution of Parvalbumin-Positive Fibers in the Mouse Brain and Their Alterations in Mouse Models of Temporal Lobe Epilepsy and Parkinson's Disease.Neurosci Bull. 2023 Nov;39(11):1683-1702. doi: 10.1007/s12264-023-01083-0. Epub 2023 Jul 31. Neurosci Bull. 2023. PMID: 37523099 Free PMC article.
-
Blockade of excitatory synaptogenesis with proximal dendrites of dentate granule cells following rapamycin treatment in a mouse model of temporal lobe epilepsy.J Comp Neurol. 2015 Feb 1;523(2):281-97. doi: 10.1002/cne.23681. Epub 2014 Oct 8. J Comp Neurol. 2015. PMID: 25234294 Free PMC article.
-
Human fetal brain-derived neural stem/progenitor cells grafted into the adult epileptic brain restrain seizures in rat models of temporal lobe epilepsy.PLoS One. 2014 Aug 8;9(8):e104092. doi: 10.1371/journal.pone.0104092. eCollection 2014. PLoS One. 2014. PMID: 25105891 Free PMC article.
References
-
- Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to spontaneous recurrent seizures in the rat lithium pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. - PubMed
-
- Babb TL, Brown WJ, Pretorius J, Davenport C, Lieb JP, Crandall PH. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984;25:729–740. - PubMed
-
- Bragin A, Engel JE, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
