Activity and expression of progesterone metabolizing 5alpha-reductase, 20alpha-hydroxysteroid oxidoreductase and 3alpha(beta)-hydroxysteroid oxidoreductases in tumorigenic (MCF-7, MDA-MB-231, T-47D) and nontumorigenic (MCF-10A) human breast cancer cells
- PMID: 12659654
- PMCID: PMC154104
- DOI: 10.1186/1471-2407-3-9
Activity and expression of progesterone metabolizing 5alpha-reductase, 20alpha-hydroxysteroid oxidoreductase and 3alpha(beta)-hydroxysteroid oxidoreductases in tumorigenic (MCF-7, MDA-MB-231, T-47D) and nontumorigenic (MCF-10A) human breast cancer cells
Abstract
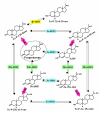
Background: Recent observations indicate that human tumorous breast tissue metabolizes progesterone differently than nontumorous breast tissue. Specifically, 5alpha-reduced metabolites (5alpha-pregnanes, shown to stimulate cell proliferation and detachment) are produced at a significantly higher rate in tumorous tissue, indicating increased 5alpha-reductase (5alphaR) activity. Conversely, the activities of 3alpha-hydroxysteroid oxidoreductase (3alpha-HSO) and 20alpha-HSO enzymes appeared to be higher in normal tissues. The elevated conversion to 5alpha-pregnanes occurred regardless of estrogen (ER) or progesterone (PR) receptor levels. To gain insight into these differences, the activities and expression of these progesterone converting enzymes were investigated in a nontumorigenic cell line, MCF-10A (ER- and PR-negative), and the three tumorigenic cell lines, MDA-MB-231 (ER- and PR-negative), MCF-7 and T-47D (ER- and PR-positive).
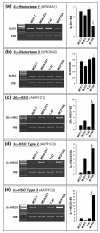
Methods: For the enzyme activity studies, either whole cells were incubated with [14C]progesterone for 2, 4, 8, and 24 hours, or the microsomal/cytosolic fraction was incubated for 15-60 minutes with [3H]progesterone, and the metabolites were identified and quantified. Semi-quantitative RT-PCR was employed to determine the relative levels of expression of 5alphaR type1 (SRD5A1), 5alphaR type 2 (SRD5A2), 20alpha-HSO (AKR1C1), 3alpha-HSO type 2 (AKR1C3), 3alpha-HSO type 3 (AKR1C2) and 3beta-HSO (HSD3B1/HSD3B2) in the four cell lines using 18S rRNA as an internal control.
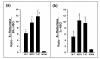
Results: The relative 5alpha-reductase activity, when considered as a ratio of 5alpha-pregnanes/4-pregnenes, was 4.21 (+/- 0.49) for MCF-7 cells, 6.24 (+/- 1.14) for MDA-MB-231 cells, 4.62 (+/- 0.43) for T-47D cells and 0.65 (+/- 0.07) for MCF-10A cells, constituting approximately 6.5-fold, 9.6-fold and 7.1 fold higher conversion to 5alpha-pregnanes in the tumorigenic cells, respectively, than in the nontumorigenic MCF-10A cells. Conversely, the 20alpha-HSO and 3alpha-HSO activities were significantly higher (p < 0.001) in MCF-10A cells than in the other three cell types. In the MCF-10A cells, 20alpha-HSO activity was 8-14-fold higher and the 3alpha-HSO activity was 2.5-5.4-fold higher than in the other three cell types. The values of 5alphaR:20alpha-HSO ratios were 16.9-32.6-fold greater and the 5alphaR:3alpha-HSO ratios were 5.2-10.5-fold greater in MCF-7, MDA-MB-231 and T-47D cells than in MCF-10A cells. RT-PCR showed significantly higher expression of 5alphaR1 (p < 0.001), and lower expression of 20alpha-HSO (p < 0.001), 3alpha-HSO2 (p < 0.001), 3alpha-HSO3 (p < 0.001) in MCF-7, MDA-MB-231 and T-47D cells than in MCF-10A cells.
Conclusion: The findings provide the first evidence that the 5alphaR activity (leading to the conversion of progesterone to the cancer promoting 5alpha-pregnanes) is significantly higher in the tumorigenic MCF-7, MDA-MB-231 and T-47D breast cell lines than in the nontumorigenic MCF-10A cell line. The higher 5alphaR activity coincides with significantly greater expression of 5alphaR1. On the other hand, the activities of 20alpha-HSO and 3alpha-HSO are higher in the MCF-10A cells than in MCF-7, MDA-MB-231 and T-47D cells; these differences in activity correlate with significantly higher expression of 20alpha-HSO, 3alpha-HSO2 and 3alpha-HSO3 in MCF-10A cells. Changes in progesterone metabolizing enzyme expression (resulting in enzyme activity changes) may be responsible for stimulating breast cancer by increased production of tumor-promoting 5alpha-pregnanes and decreased production of anti-cancer 20alpha--and 3alpha-4-pregnenes.
Figures

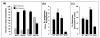

Similar articles
-
Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma.BMC Cancer. 2004 Jun 22;4:27. doi: 10.1186/1471-2407-4-27. BMC Cancer. 2004. PMID: 15212687 Free PMC article.
-
Dutasteride affects progesterone metabolizing enzyme activity/expression in human breast cell lines resulting in suppression of cell proliferation and detachment.J Steroid Biochem Mol Biol. 2006 Aug;100(4-5):129-40. doi: 10.1016/j.jsbmb.2006.03.010. Epub 2006 Jun 27. J Steroid Biochem Mol Biol. 2006. PMID: 16806904
-
Progesterone metabolites in breast cancer.Endocr Relat Cancer. 2006 Sep;13(3):717-38. doi: 10.1677/erc.1.01010. Endocr Relat Cancer. 2006. PMID: 16954427 Review.
-
The 4-pregnene and 5alpha-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion.Cancer Res. 2000 Feb 15;60(4):936-43. Cancer Res. 2000. PMID: 10706108
-
The dialectic role of progesterone.Maturitas. 2009 Apr 20;62(4):326-9. doi: 10.1016/j.maturitas.2008.12.009. Epub 2009 Jan 25. Maturitas. 2009. PMID: 19171445 Review.
Cited by
-
Progesterone metabolites regulate induction, growth, and suppression of estrogen- and progesterone receptor-negative human breast cell tumors.Breast Cancer Res. 2013 May 11;15(3):R38. doi: 10.1186/bcr3422. Breast Cancer Res. 2013. PMID: 25927181 Free PMC article.
-
Progesterone induces progesterone receptor gene (PGR) expression via rapid activation of protein kinase pathways required for cooperative estrogen receptor alpha (ER) and progesterone receptor (PR) genomic action at ER/PR target genes.Steroids. 2016 Oct;114:48-58. doi: 10.1016/j.steroids.2016.09.004. Epub 2016 Sep 15. Steroids. 2016. PMID: 27641443 Free PMC article.
-
Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma.BMC Cancer. 2004 Jun 22;4:27. doi: 10.1186/1471-2407-4-27. BMC Cancer. 2004. PMID: 15212687 Free PMC article.
-
Divergent control of Cav-1 expression in non-cancerous Li-Fraumeni syndrome and human cancer cell lines.Cancer Biol Ther. 2013 Jan;14(1):29-38. doi: 10.4161/cbt.22621. Epub 2012 Oct 31. Cancer Biol Ther. 2013. PMID: 23114650 Free PMC article.
-
Co-Adjuvant Therapy Efficacy of Catechin and Procyanidin B2 with Docetaxel on Hormone-Related Cancers In Vitro.Int J Mol Sci. 2021 Jul 2;22(13):7178. doi: 10.3390/ijms22137178. Int J Mol Sci. 2021. PMID: 34281228 Free PMC article.
References
-
- Wiebe JP, Muzia D, Hu J, Szwajcer D, Hill SA, Seachrist JL. The 4-pregnene and 5α-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res. 2000;60:936–943. - PubMed
-
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. - PubMed
-
- Anderson WA, Perotti ME, McManaway M, Lindsey S, Eckberg WR. Similarities and differences in the ultrastructure of two hormone-dependent and one independent human breast carcinoma grown in athymic nude mice: comparison with the rat DMBA-induced tumor and normal secretory mammocytes. J Submicrosc Cytol. 1984;16:673–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

