Contributions of cell metabolism and H+ diffusion to the acidic pH of tumors
- PMID: 12659686
- PMCID: PMC1502399
- DOI: 10.1016/s1476-5586(03)80005-2
Contributions of cell metabolism and H+ diffusion to the acidic pH of tumors
Abstract
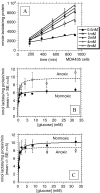
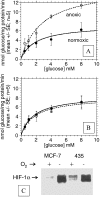
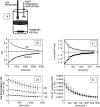
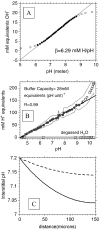
The tumor microenvironment is hypoxic and acidic. These conditions have a significant impact on tumor progression and response to therapies. There is strong evidence that tumor hypoxia results from inefficient perfusion due to a chaotic vasculature. Consequently, some tumor regions are well oxygenated and others are hypoxic. It is commonly believed that hypoxic regions are acidic due to a stimulation of glycolysis through hypoxia, yet this is not yet demonstrated. The current study investigates the causes of tumor acidity by determining acid production rates and the mechanism of diffusion for H(+) equivalents through model systems. Two breast cancer cell lines were investigated with divergent metabolic profiles: nonmetastatic MCF-7/s and highly metastatic MDA-mb-435 cells. Glycolysis and acid production are inhibited by oxygen in MCF-7/s cells, but not in MDA-mb-435 cells. Tumors of MDA-mb-435 cells are significantly more acidic than are tumors of MCF-7/s cells, suggesting that tumor acidity is primarily caused by endogenous metabolism, and not the lack of oxygen. Metabolically produced protons are shown to diffuse in association with mobile buffers, in concordance with previous studies. The metabolic and diffusion data were analyzed using a reaction-diffusion model to demonstrate that the consequent pH profiles conform well to measured pH values for tumors of these two cell lines.
Figures


References
-
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [Review; 214 references] - PubMed
-
- Okunieff P, Dols S, Lee J, Singer S, Vaupel P, Neuringer LJ, Beshah K. Angiogenesis determines blood flow, metabolism, growth rate, and ATPase kinetics of tumors growing in an irradiated bed: 31P and 2H nuclear magnetic resonance studies. Cancer Res. 1991;51:3289–3295. - PubMed
-
- Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2:343–366. - PubMed
-
- Gillies RJ, Liu Z, Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994;267:t-203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
