A mathematical model for cisplatin cellular pharmacodynamics
- PMID: 12659689
- PMCID: PMC1502402
- DOI: 10.1016/s1476-5586(03)80008-8
A mathematical model for cisplatin cellular pharmacodynamics
Abstract
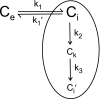
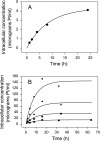
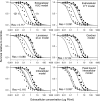
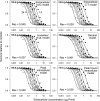
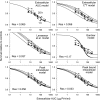
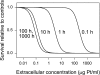
A simple theoretical model for the cellular pharmacodynamics of cisplatin is presented. The model, which takes into account the kinetics of cisplatin uptake by cells and the intracellular binding of the drug, can be used to predict the dependence of survival (relative to controls) on the time course of extracellular exposure. Cellular pharmacokinetic parameters are derived from uptake data for human ovarian and head and neck cancer cell lines. Survival relative to controls is assumed to depend on the peak concentration of DNA-bound intracellular platinum. Model predictions agree well with published data on cisplatin cytotoxicity for three different cancer cell lines, over a wide range of exposure times. In comparison with previously published mathematical models for anticancer drug pharmacodynamics, the present model provides a better fit to experimental data sets including long exposure times (approximately 100 hours). The model provides a possible explanation for the fact that cell kill correlates well with area under the extracellular concentration-time curve in some data sets, but not in others. The model may be useful for optimizing delivery schedules and for the dosing of cisplatin for cancer therapy.
Figures
Similar articles
-
Two-mechanism peak concentration model for cellular pharmacodynamics of Doxorubicin.Neoplasia. 2005 Jul;7(7):705-13. doi: 10.1593/neo.05118. Neoplasia. 2005. PMID: 16026650 Free PMC article.
-
Platinum drug effects on the expression of genes in the polyamine pathway: time-course and concentration-effect analysis based on Affymetrix gene expression profiling of A2780 ovarian carcinoma cells.Cancer Chemother Pharmacol. 2007 May;59(6):711-23. doi: 10.1007/s00280-006-0325-3. Epub 2006 Sep 22. Cancer Chemother Pharmacol. 2007. PMID: 17021820
-
Enhanced antitumor activity of irofulven in combination with 5-fluorouracil and cisplatin in human colon and ovarian carcinoma cells.Int J Oncol. 2003 Nov;23(5):1347-55. Int J Oncol. 2003. PMID: 14532976
-
Establishment and characterization of an in vitro model of acquired resistance to cisplatin in a human testicular nonseminomatous germ cell line.Cancer Res. 1992 Apr 1;52(7):1710-6. Cancer Res. 1992. PMID: 1312897
-
Comparison of cellular accumulation and cytotoxicity of cisplatin with that of tetraplatin and amminedibutyratodichloro(cyclohexylamine)platinum(IV) (JM221) in human ovarian carcinoma cell lines.Cancer Res. 1992 Nov 15;52(22):6188-93. Cancer Res. 1992. PMID: 1423261
Cited by
-
Two-mechanism peak concentration model for cellular pharmacodynamics of Doxorubicin.Neoplasia. 2005 Jul;7(7):705-13. doi: 10.1593/neo.05118. Neoplasia. 2005. PMID: 16026650 Free PMC article.
-
Computational approaches to analyse and predict small molecule transport and distribution at cellular and subcellular levels.Biopharm Drug Dispos. 2014 Jan;35(1):15-32. doi: 10.1002/bdd.1879. Epub 2013 Dec 10. Biopharm Drug Dispos. 2014. PMID: 24218242 Free PMC article. Review.
-
Mathematical Based Calculation of Drug Penetration Depth in Solid Tumors.Biomed Res Int. 2016;2016:8437247. doi: 10.1155/2016/8437247. Epub 2016 Jun 8. Biomed Res Int. 2016. PMID: 27376087 Free PMC article.
-
Dynamic Phenotypic Switching and Group Behavior Help Non-Small Cell Lung Cancer Cells Evade Chemotherapy.Biomolecules. 2021 Dec 21;12(1):8. doi: 10.3390/biom12010008. Biomolecules. 2021. PMID: 35053156 Free PMC article.
-
Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study.Lancet Child Adolesc Health. 2021 Apr;5(4):274-283. doi: 10.1016/S2352-4642(21)00020-1. Epub 2021 Feb 12. Lancet Child Adolesc Health. 2021. PMID: 33581749 Free PMC article.
References
-
- Reed E, Dabholkar M, Chabner BA. Cancer Chemotherapy and Biotherapy. Philadelphia: Lippincott-Raven; 1996. Platinum analogues.
-
- Troger V, Fischel JL, Formento P, Gioanni J, Milano G. Effects of prolonged exposure to cisplatin on cytotoxicity and intracellular drug concentration. Eur J Cancer. 1992;28:82–86. - PubMed
-
- Kurihara N, Kubota T, Hoshiya Y, Otani Y, Kumai K, Kitajima M. Antitumor activity of cis-diaminedichloroplatinum (II) depends on its time x concentration product against human gastric cancer cell lines in vitro. J Surg Oncol. 1995;60:238–241. - PubMed
-
- Drewinko B, Gottlieb JA. Action of cis-dichlorodiamineplatinum(II) (NSC-119875) at the cellular level. Cancer Chemother Rep. 1975;59:665–673. - PubMed
-
- Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, Takiyama W, Ishida K, Isono K, Makuuchi H, Imamura M, Shinoda M, Ikeuchi S, Kabuto T, Yamana H, Fukuda H. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407) Jpn J Clin Oncol. 2001;31:419–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
