Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening
- PMID: 12660152
- PMCID: PMC152903
- DOI: 10.1093/emboj/cdg159
Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening
Abstract
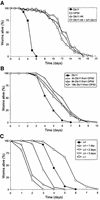
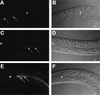
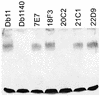
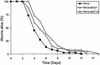
The human opportunistic pathogen Serratia marcescens is a bacterium with a broad host range, and represents a growing problem for public health. Serratia marcescens kills Caenorhabditis elegans after colonizing the nematode's intestine. We used C.elegans to screen a bank of transposon-induced S.marcescens mutants and isolated 23 clones with an attenuated virulence. Nine of the selected bacterial clones also showed a reduced virulence in an insect model of infection. Of these, three exhibited a reduced cytotoxicity in vitro, and among them one was also markedly attenuated in its virulence in a murine lung infection model. For 21 of the 23 mutants, the transposon insertion site was identified. This revealed that among the genes necessary for full in vivo virulence are those that function in lipopolysaccharide (LPS) biosynthesis, iron uptake and hemolysin production. Using this system we also identified novel conserved virulence factors required for Pseudomonas aeruginosa pathogenicity. This study extends the utility of C.elegans as an in vivo model for the study of bacterial virulence and advances the molecular understanding of S.marcescens pathogenicity.
Figures




References
-
- Aballay A. and Ausubel,F.M. (2002) Caenorhabditis elegans as a host for the study of host–pathogen interactions. Curr. Opin. Microbiol., 5, 97–101. - PubMed
-
- Alexandrakis G., Alfonso,E.C. and Miller,D. (2000) Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology, 107, 1497–1502. - PubMed
-
- Altschul S.F., Gish,W., Miller,W., Myers,E.W. and Lipman,D.J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

