p53 activates ICAM-1 (CD54) expression in an NF-kappaB-independent manner
- PMID: 12660163
- PMCID: PMC152901
- DOI: 10.1093/emboj/cdg157
p53 activates ICAM-1 (CD54) expression in an NF-kappaB-independent manner
Abstract
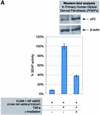
Intercellular adhesion molecule-1 (ICAM-1) is a crucial receptor in the cell-cell interaction, a process central to the reaction to all forms of injury. Its expression is upregulated in response to a variety of inflammatory/immune mediators, including cellular stresses. The NF-kappaB signalling pathway is known to be important for activation of ICAM-1 transcription. Here we demonstrate that ICAM-1 induction represents a new cellular response to p53 activation and that NF-kappaB inhibition does not prevent the effect of p53 on ICAM-1 expression after DNA damage. Induction of ICAM-1 is abolished after treatment with the specific p53 inhibitor pifithrin-alpha and is abrogated in p53-deficient cell lines. Furthermore, we map two functional p53-responsive elements to the introns of the ICAM-1 gene, and show that they confer inducibility to p53 in a fashion similar to other p53 target genes. These results support an NF-kappaB-independent role for p53 in ICAM-1 regulation that may link p53 to ICAM-1 function in various physiological and pathological settings.
Figures











References
-
- Arnould T., Michiels,C. and Remacle,J. (1993) Increased PMN adherence on endothelial cells after hypoxia: involvement of PAF, CD18/CD11b and ICAM-1. Am. J. Physiol., 264, C1102–C1110. - PubMed
-
- Avantaggiati M.L., Ogryzko,V., Gardner,K., Giordano,A., Levine,A.S. and Kelly,K. (1997) Recruitment of p300/CBP in p53-dependent signal pathways. Cell, 89, 1175–1184. - PubMed
-
- Baeuml H., Behrends,U., Peter,R.U., Mueller,S., Kammerbauer,C., Caughman,S.W. and Degitz,K. (1997) Ionizing radiation induces, via generation of reactive oxygen intermediates, intercellular adhesion molecule-1 (ICAM-1) gene transcription and NF κB-like binding activity in the ICAM-1 transcriptional regulatory region. Free Radical Res., 27, 127–142. - PubMed
-
- Bourdillon M.C., Poston,R.N., Covacho,C., Chignier,E., Bricca,G. and McGregor,J.L. (2000) ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE(–/–)/ICAM-1(–/–)) fed a fat or a chow diet. Arterioscler. Thromb. Vasc. Biol., 20, 2630–2635. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

