ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation
- PMID: 12660167
- PMCID: PMC152910
- DOI: 10.1093/emboj/cdg166
ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation
Abstract
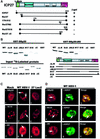
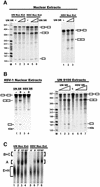
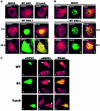
Infection with some viruses can alter cellular mRNA processing to favor viral gene expression. We present evidence that herpes simplex virus 1 (HSV-1) protein ICP27, which contributes to host shut-off by inhibiting pre-mRNA splicing, interacts with essential splicing factors termed SR proteins and affects their phosphorylation. During HSV-1 infection, phosphorylation of several SR proteins was reduced and this correlated with a subnuclear redistribution. Exogenous SR proteins restored splicing in ICP27-inhibited nuclear extracts and SR proteins isolated from HSV-1-infected cells activated splicing in uninfected S100 extracts, indicating that inhibition occurs by a reversible mechanism. Spliceosome assembly was blocked at the pre-spliceosomal complex A stage. Furthermore, we show that ICP27 interacts with SRPK1 and relocalizes it to the nucleus; moreover, SRPK1 activity was altered in the presence of ICP27 in vitro. We propose that ICP27 modifies SRPK1 activity resulting in hypophosphorylation of SR proteins impairing their ability to function in spliceosome assembly.
Figures




Similar articles
-
Molecular Mechanism of SR Protein Kinase 1 Inhibition by the Herpes Virus Protein ICP27.mBio. 2019 Oct 22;10(5):e02551-19. doi: 10.1128/mBio.02551-19. mBio. 2019. PMID: 31641093 Free PMC article.
-
Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27.Virology. 2002 Mar 1;294(1):189-98. doi: 10.1006/viro.2001.1301. Virology. 2002. PMID: 11886277
-
Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect.J Virol. 1994 Dec;68(12):7790-9. doi: 10.1128/JVI.68.12.7790-7799.1994. J Virol. 1994. PMID: 7966568 Free PMC article.
-
Properties of an HSV-1 regulatory protein that appears to impair host cell splicing.Infect Agents Dis. 1994 Apr-Jun;3(2-3):59-67. Infect Agents Dis. 1994. PMID: 7812656 Review.
-
The many roles of the regulatory protein ICP27 during herpes simplex virus infection.Front Biosci. 2008 May 1;13:5241-56. doi: 10.2741/3078. Front Biosci. 2008. PMID: 18508584 Review.
Cited by
-
Design of a covalent protein-protein interaction inhibitor of SRPKs to suppress angiogenesis and invasion of cancer cells.Commun Chem. 2024 Jun 27;7(1):144. doi: 10.1038/s42004-024-01230-2. Commun Chem. 2024. PMID: 38937565 Free PMC article.
-
Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27.J Virol. 2005 Apr;79(7):4120-31. doi: 10.1128/JVI.79.7.4120-4131.2005. J Virol. 2005. PMID: 15767413 Free PMC article.
-
ICP27 interacts with the C-terminal domain of RNA polymerase II and facilitates its recruitment to herpes simplex virus 1 transcription sites, where it undergoes proteasomal degradation during infection.J Virol. 2006 Apr;80(7):3567-81. doi: 10.1128/JVI.80.7.3567-3581.2006. J Virol. 2006. PMID: 16537625 Free PMC article.
-
Herpes simplex virus-1 induces expression of a novel MxA isoform that enhances viral replication.Immunol Cell Biol. 2011 Feb;89(2):173-82. doi: 10.1038/icb.2010.83. Epub 2010 Jul 6. Immunol Cell Biol. 2011. PMID: 20603636 Free PMC article.
-
Varicella-zoster virus IE4 protein interacts with SR proteins and exports mRNAs through the TAP/NXF1 pathway.PLoS One. 2009 Nov 18;4(11):e7882. doi: 10.1371/journal.pone.0007882. PLoS One. 2009. PMID: 19924249 Free PMC article.
References
-
- Champion-Arnaud P. and Reed,R. (1994) The prespliceosome components SAP 49 and SAP 145 interact in a complex implicated in tethering U2 snRNP to the branch site. Genes Dev., 8, 1974–1983. - PubMed
-
- Fu X.D. and Maniatis,T. (1990) Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature, 343, 437–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

