Progesterone increases susceptibility and decreases immune responses to genital herpes infection
- PMID: 12663762
- PMCID: PMC152159
- DOI: 10.1128/jvi.77.8.4558-4565.2003
Progesterone increases susceptibility and decreases immune responses to genital herpes infection
Abstract
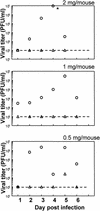
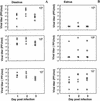
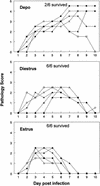
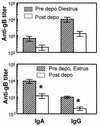
Depo-provera, a long-acting progestational formulation, is widely used to facilitate infection of sexually transmitted diseases in animal models. We have previously reported that hormone treatments change susceptibility and immune responses to genital tract infections. In this study we compared the changes in susceptibility of mice to genital herpes simplex virus type 2 (HSV-2) after Depo-provera or a saline suspension of progesterone (P-sal). We found that following Depo-provera-treatment, mice had prolonged diestrus that lasted more than 4 weeks. This coincided with a 100-fold increase in susceptibility to genital HSV-2 compared to that of untreated mice. Mice given P-sal were in diestrous stage for 4 to 6 days before returning to irregular reproductive cycles. When these mice were infected at diestrus they showed a 10-fold increase in susceptibility compared to that of normal, untreated mice. P-sal-treated mice infected at estrus were susceptible to HSV-2, depending on the infectious dose. Normal, untreated mice in estrus were not susceptible to HSV-2, even at a high infectious dose of 10(7) PFU. In addition to alterations in susceptibility, Depo-provera treatment had inhibitory effects on immune responses to HSV-2. Mice immunized with HSV-2 protein (gB) and treated with Depo-provera showed significant lowering of local HSV-2-specific immunoglobulin G (IgG) and IgA in their vaginal washes. Mice immunized with an attenuated strain of HSV-2 2 weeks after Depo-provera treatment failed to develop protection when challenged intravaginally with wild-type HSV-2. In contrast, mice given progesterone and immunized at diestrus or estrus were completely protected from intravaginal challenge. These studies show that Depo-provera treatment changes susceptibility and local immune responses to genital HSV-2 infection. Animal models and vaccine strategies for sexually transmitted diseases need to consider the effect of hormone treatments on susceptibility and immune responses.
Figures



References
-
- Gallichan, W. S., T. Gurasachi, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligonucleotides as an adjuvant dramatically increases IgA and protection against HSV-2 in the genital tract. J. Immunol. 166:3451-3457. - PubMed
-
- Gallichan, W. S., and K. L. Rosenthal. 1996. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 224:487-497. - PubMed
-
- Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. - PubMed
-
- Kaushic, C., E. Frauendorf, R. M. Rossoll, J. M. Richardson, and C. R. Wira. 1998. Influence of estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am. J. Reprod. Immunol. 39:209-216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

