Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis
- PMID: 12663771
- PMCID: PMC152115
- DOI: 10.1128/jvi.77.8.4646-4657.2003
Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis
Abstract
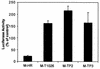
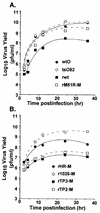
The vesicular stomatitis virus (VSV) matrix (M) protein plays a major role in the virus-induced inhibition of host gene expression. It has been proposed that the inhibition of host gene expression by M protein is responsible for suppressing activation of host interferon gene expression. Most wild-type (wt) strains of VSV induce little if any interferon gene expression. Interferon-inducing mutants of VSV have been isolated previously, many of which contain mutations in their M proteins. However, it was not known whether these M protein mutations were responsible for the interferon-inducing phenotype of these viruses. Alternatively, mutations in other genes besides the M gene may enhance the ability of VSV to induce interferons. These hypotheses were tested by transfecting cells with mRNA expressing wt and mutant M proteins in the absence of other viral components and determining their ability to inhibit interferon gene expression. The M protein mutations were the M51R mutation originally found in the tsO82 and T1026R1 mutant viruses, the double substitution V221F and S226R found in the TP3 mutant virus, and the triple substitution E213A, V221F, and S226R found in the TP2 mutant virus. wt M proteins suppressed expression of luciferase from the simian virus 40 promoter and from the beta interferon (IFN-beta) promoter, while M proteins of interferon-inducing viruses were unable to inhibit luciferase expression from either promoter. The M genes of the interferon-inducing mutants of VSV were incorporated into the wt background of a recombinant VSV infectious cDNA clone. The resulting recombinant viruses were tested for their ability to activate interferon gene expression and for their ability to inhibit host RNA and protein synthesis. Each of the recombinant viruses containing M protein mutations induced expression of a luciferase reporter gene driven by the IFN-beta promoter and induced production of interferon bioactivity more effectively than viruses containing wt M proteins. Furthermore, the M protein mutant viruses were defective in their ability to inhibit both host RNA synthesis and host protein synthesis. These data support the idea that wt M protein suppresses interferon gene expression through the general inhibition of host RNA and protein synthesis.
Figures





References
-
- Ahmed, M., and D. S. Lyles. 1997. Identification of a consensus mutation in M protein of vesicular stomatitis virus from persistently infected cells that affects inhibition of host-directed gene expression. Virology 237:378-383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

