Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55(gag) associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100
- PMID: 12663787
- PMCID: PMC152122
- DOI: 10.1128/jvi.77.8.4805-4817.2003
Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55(gag) associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100
Abstract
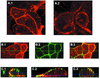
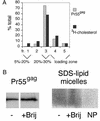
The assembly and budding of human immunodeficiency virus type 1 (HIV-1) at the plasma membrane are directed by the viral core protein Pr55(gag). We have analyzed whether Pr55(gag) has intrinsic affinity for sphingolipid- and cholesterol-enriched raft microdomains at the plasma membrane. Pr55(gag) has previously been reported to associate with Triton X-100-resistant rafts, since both intracellular membranes and virus-like Pr55(gag) particles (VLPs) yield buoyant Pr55(gag) complexes upon Triton X-100 extraction at cold temperatures, a phenotype that is usually considered to indicate association of a protein with rafts. However, we show here that the buoyant density of Triton X-100-treated Pr55(gag) complexes cannot be taken as a proof for raft association of Pr55(gag), since lipid analyses of Triton X-100-treated VLPs demonstrated that the detergent readily solubilizes the bulk of membrane lipids from Pr55(gag). However, Pr55(gag) might nevertheless be a raft-associated protein, since confocal fluorescence microscopy indicated that coalescence of GM1-positive rafts at the cell surface led to copatching of membrane-bound Pr55(gag). Furthermore, extraction of intracellular membranes or VLPs with Brij98 yielded buoyant Pr55(gag) complexes of low density. Lipid analyses of Brij98-treated VLPs suggested that a large fraction of the envelope cholesterol and phospholipids was resistant to Brij98. Collectively, these results suggest that Pr55(gag) localizes to membrane microdomains that are largely resistant to Brij98 but sensitive to Triton X-100, and these membrane domains provide the platform for assembly and budding of Pr55(gag) VLPs.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

