Complexes of poliovirus serotypes with their common cellular receptor, CD155
- PMID: 12663789
- PMCID: PMC152153
- DOI: 10.1128/jvi.77.8.4827-4835.2003
Complexes of poliovirus serotypes with their common cellular receptor, CD155
Abstract
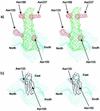
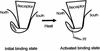
Structures of all three poliovirus (PV) serotypes (PV1, PV2, and PV3) complexed with their cellular receptor, PV receptor (PVR or CD155), were determined by cryoelectron microscopy. Both glycosylated and fully deglycosylated CD155 exhibited similar binding sites and orientations in the viral canyon for all three PV serotypes, showing that all three serotypes use a common mechanism for cell entry. Difference maps between the glycosylated and deglycosylated CD155 complexes determined the sites of the carbohydrate moieties that, in turn, helped to verify the position of the receptor relative to the viral surface. The proximity of the CD155 carbohydrate site at Asn105 to the viral surface in the receptor-virus complex suggests that it might interfere with receptor docking, an observation consistent with the properties of mutant CD155. The footprints of CD155 on PV surfaces indicate that the south rim of the canyon dominates the virus-receptor interactions and may correspond to the initial CD155 binding state of the receptor-mediated viral uncoating. In contrast, the interaction of CD155 with the north rim of the canyon, especially the region immediately outside the viral hydrophobic pocket that normally binds a cellular "pocket factor," may be critical for the release of the pocket factor, decreasing the virus stability and hence initiating uncoating. The large area of the CD155 footprint on the PV surface, in comparison with other picornavirus-receptor interactions, could be a potential limitation on the viability of PV escape mutants from antibody neutralization. Many of these are likely to have lost their ability to bind CD155, resulting in there being only three PV serotypes.
Figures


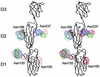

References
-
- Baker, T. S., and R. H. Cheng. 1996. A model-based approach for determining orientations of biological macromolecules imaged by cryoelectron microscopy. J. Struct. Biol. 116:120-130. - PubMed
-
- Bernhardt, G., J. A. Bibb, J. Bradley, and E. Wimmer. 1994. Molecular characterization of the cellular receptor for poliovirus. Virology 199:105-113. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

