Binding of adenovirus capsid to dipalmitoyl phosphatidylcholine provides a novel pathway for virus entry
- PMID: 12663792
- PMCID: PMC152149
- DOI: 10.1128/jvi.77.8.4858-4866.2003
Binding of adenovirus capsid to dipalmitoyl phosphatidylcholine provides a novel pathway for virus entry
Abstract
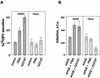
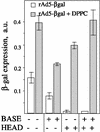
Adenovirus (Ad) is an airborne, nonenveloped virus infecting respiratory epithelium. To study the mechanism of Ad entry, we used alveolar adenocarcinoma A549 cells, which have retained the ability of alveolar epithelial type II cells to synthesize the major component of pulmonary surfactant, disaturated phosphatidylcholine. Stimulation of phosphatidylcholine secretion by calcium ionophore or phorbol ester augmented the susceptibility of these cells to Ad. Both Ad infection and recombinant-Ad-mediated transfection increased in the presence of dipalmitoyl phosphatidylcholine (DPPC) liposomes in culture medium. Importantly, in the presence of DPPC liposomes, virus penetrates the cells independently of virus-specific protein receptors. DPPC vesicles bind Ad and are efficiently incorporated by A549 lung cells, serving as a virus vehicle during Ad penetration. To identify the viral protein(s) mediating Ad binding, a flotation of liposomes preincubated with structural viral proteins was employed, showing that the only Ad protein bound to DPPC vesicles was a hexon. The hexon preserved its phospholipid-binding properties upon purification, confirming its involvement in virus binding to the phospholipid. Given that disaturated phosphatidylcholine not only covers the inner surface of alveoli in the lungs but also reenters alveolar epithelium during lung surfactant turnover, Ad binding to this phospholipid may provide a pathway for virus entry into alveolar epithelium in vivo.
Figures


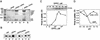



References
-
- Arcasoy, S. M., J. Latoche, M. Gondor, S. C. Watkins, R. A. Henderson, R. Hughey, O. J. Finn, and J. M. Pilewski. 1997. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am. J. Respir. Cell. Mol. Biol. 17:422-435. - PubMed
-
- Benson, S. D., J. K. Bamford, D. H. Bamford, and R. M. Burnett. 2002. The X-ray crystal structure of P3, the major coat protein of the lipid-containing bacteriophage PRD1, at 1.65 Ä resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 58:39-59. - PubMed
-
- Benson, S. D., J. K. H. Bamford, D. H. Bamford, and R. M. Burnett. 1999. Viral evolution revealed by bacteriophage PRD1 and human adenovirus coat protein structures. Cell 98:825-833. - PubMed
-
- Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

