Latent membrane protein 1 inhibits Epstein-Barr virus lytic cycle induction and progress via different mechanisms
- PMID: 12663807
- PMCID: PMC152124
- DOI: 10.1128/jvi.77.8.5000-5007.2003
Latent membrane protein 1 inhibits Epstein-Barr virus lytic cycle induction and progress via different mechanisms
Abstract
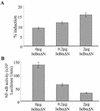
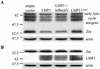
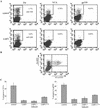
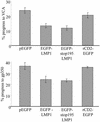
Epstein-Barr virus (EBV) is a potent growth-transforming agent of human B cells. It has previously been shown that viral latent membrane protein 1 (LMP1) is essential for EBV-induced transformation of normal B cells and contributes to maintenance of latency in vitro. Using the EBV-positive Burkitt's lymphoma line P3HR1-c16, which lacks LMP1 during latency and which can readily be activated into virus-productive lytic cycle, we found that LMP1 inhibits lytic cycle induction via the transcription factor NF-kappa B. In addition, LMP1 inhibits lytic cycle progress via two distinct NF-kappa B-independent mechanisms: one involving the cytosolic C-terminal activating regions and the other involving the transmembrane region of LMP1. These findings indicate that in B cells EBV self-limits its lytic cycle via three distinct LMP1-mediated mechanisms.
Figures


References
-
- Arenzana-Seisdedos, F., B. Fernandez, I. Dominguez, J. M. Jacque, D. Thomas, M. T. Diaz-Meco, J. Moscat, and J. L. Virelizier. 1993. Phosphatidylcholine hydrolysis activates NF-κB and increases human immunodeficiency virus replication in human monocytes and T lymphocytes. J. Virol. 67:6596-6604. - PMC - PubMed
-
- Berberich, I., G. L. Shu, and E. A. Clark. 1994. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J. Immunol. 153:4357-4366. - PubMed
-
- Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

