The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans
- PMID: 12663858
- PMCID: PMC153647
- DOI: 10.1073/pnas.0730053100
The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans
Abstract
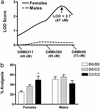
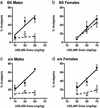
Sex specificity of neural mechanisms modulating nociceptive information has been demonstrated in rodents, and these qualitative sex differences appear to be relevant to analgesia from kappa-opioid receptor agonists, a drug class reported to be clinically effective only in women. Via quantitative trait locus mapping followed by a candidate gene strategy using both mutant mice and pharmacological tools, we now demonstrate that the melanocortin-1 receptor (Mc1r) gene mediates kappa-opioid analgesia in female mice only. This finding suggested that individuals with variants of the human MC1R gene, associated in our species with red hair and fair skin, might also display altered kappa-opioid analgesia. We found that women with two variant MC1R alleles displayed significantly greater analgesia from the kappa-opioid, pentazocine, than all other groups. This study demonstrates an unexpected role for the MC1R gene, verifies that pain modulation in the two sexes involves neurochemically distinct substrates, and represents an example of a direct translation of a pharmacogenetic finding from mouse to human.
Figures
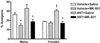

References
-
- Berkley K J. Behav Brain Sci. 1997;20:371–380. - PubMed
-
- Liu N-J, Gintzler A R. Pain. 2000;85:273–281. - PubMed
-
- Tershner S A, Mitchell J M, Fields H L. Pain. 2000;85:153–159. - PubMed
-
- Mogil J S, Sternberg W F, Kest B, Marek P, Liebeskind J C. Pain. 1993;53:17–25. - PubMed
-
- Kavaliers M, Galea L A M. Pain. 1995;63:327–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

