c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor
- PMID: 12665585
- PMCID: PMC152565
- DOI: 10.1128/MCB.23.8.2871-2882.2003
c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor
Abstract
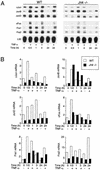
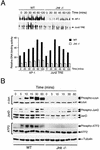
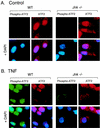
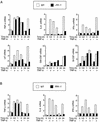
The c-Jun NH(2)-terminal kinase (JNK) is activated by the cytokine tumor necrosis factor (TNF). This pathway is implicated in the regulation of AP-1-dependent gene expression by TNF. To examine the role of the JNK signaling pathway, we compared the effects of TNF on wild-type and Jnk1(-/-) Jnk2(-/-) murine embryo fibroblasts. We show that JNK is required for the normal regulation of AP-1 by TNF. The JNK-deficient cells exhibited decreased expression of c-Jun, JunD, c-Fos, Fra1, and Fra2; decreased phosphorylation of c-Jun and JunD; and decreased AP-1 DNA binding activity. The JNK-deficient cells also exhibited defects in the regulation of the AP-1-related transcription factor ATF2. These changes were associated with marked defects in TNF-regulated gene expression. The JNK signal transduction pathway is therefore essential for AP-1 transcription factor regulation in cells exposed to TNF.
Figures
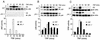


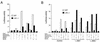
References
-
- Baud, V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11:372-377. - PubMed
-
- Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. - PMC - PubMed
-
- Brenner, D. A., M. O'Hara, P. Angel, M. Chojkier, and M. Karin. 1989. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature 337:661-663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
