Role of nitric oxide in hypoxic cerebral vasodilatation in the ovine fetus
- PMID: 12665609
- PMCID: PMC2342960
- DOI: 10.1113/jphysiol.2002.038034
Role of nitric oxide in hypoxic cerebral vasodilatation in the ovine fetus
Abstract
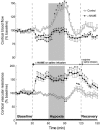
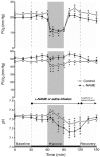
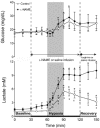
To investigate the role of nitric oxide (NO) in fetal cerebral circulatory responses to acute hypoxia, near-term fetal sheep were instrumented with laser Doppler probes placed in the parasagittal parietal cortices and vascular catheters in the sagittal sinus and brachiocephalic artery. After a 3 day recovery period, responses of cortical blood flow (CBF) to hypoxia were compared with and without inhibition of nitric oxide synthase (NOS). After an initial 30 min baseline period, fetuses were given a bolus followed by a continuous infusion of Nomega-nitro-L-arginine methyl ester (L-NAME), or saline vehicle as control. After administration of L-NAME, CBF decreased by 14 +/- 6 % (P < 0.01) despite increases in arterial blood pressure of 15 mmHg, resulting in an ~60 % increase in cerebrovascular resistance. Thirty minutes following initiation of L-NAME or vehicle infusion, fetal systemic hypoxia was induced by allowing the ewes to breathe 10-11 % oxygen. In control fetuses CBF increased progressively to 145 +/- 9 % of baseline (P < 0.01) after 30 min, while cortical release of cyclic guanylate cyclase (cGMP), an index of NOS activity, increased 26 +/- 8 % (P < 0.05). In contrast, CBF in L-NAME-treated fetuses increased to only 115 % of the reduced CBF baseline, whereas cortical release of cGMP did not change significantly. In summary, basal levels of NO lower resting cortical vascular resistance by ~15 % in the fetal sheep. Inhibition of NO synthesis attenuates hypoxic cerebral relaxation but does not completely prevent the characteristic increases in CBF. Hypoxic increases in NO directly increase cortical production of cGMP and inhibition of NO synthesis ablates these changes in cGMP.
Figures



References
-
- Armstead WM. Role of nitric oxide, cyclic nucleotides, and the activation of ATP-sensitive K+ channels in the contribution of adenosine to hypoxia-induced pial artery dilation. J Cereb Blood Flow Metab. 1997;17:100–108. - PubMed
-
- Buchanan JE, Phillis JW. The role of nitric oxide in the regulation of cerebral blood flow. Brain Res. 1993;610:248–255. - PubMed
-
- Faraci FM. Role of endothelium-derived relaxing factor in cerebral circulation: large arteries vs. microcirculation. Am J Physiol. 1991;261:H1038–1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

