Generation and characterization of chimeric recombinant AAV vectors
- PMID: 12668138
- PMCID: PMC2636682
- DOI: 10.1016/s1525-0016(03)00012-1
Generation and characterization of chimeric recombinant AAV vectors
Abstract
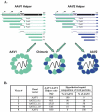
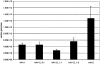
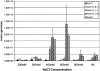
Although most animal experiments with recombinant adeno-associated virus (AAV) vectors have been based on AAV serotype 2, recent studies showed that AAV vectors based on AAV serotype 1 performed more efficiently in muscle and other tissues. On the other hand, AAV2-based vectors can be readily purified by heparin column. To combine the advantages of both types of vectors, we developed a strategy to generate chimeric vectors by using a mixture of AAV helper plasmids encoding both serotypes in the transfection process. Because the AAV packaging machinery cannot distinguish between closely related AAV1 and AAV2 capsid proteins, each packaged virion contains capsid proteins from both serotypes. As expected, the resulting chimeric vectors could be purified by heparin column. Neutralizing antibody assays showed that the chimeric vectors can be inhibited by either AAV1 or AAV2 antiserum. In vivo, the chimeric vectors direct levels of expression similar to those of AAV1 in muscle or AAV2 in liver; that is, they combine the best transduction characteristics of both parent vectors. In summary, this study provides a straightforward method for combining various properties of different AAV serotypes into one vector. Potential limitations of the chimeric vectors are also discussed.
Figures
References
-
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 1992;158:97–129. - PubMed
-
- Berns KI. Parvoviridae. In: Murphy FA, et al., editors. Virus Taxonomy: The Sixth Report of the International Committee on Taxonomy of Viruses. Springer Verlag; Vienna/New York: 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

