The endogenous calcium ions of horseradish peroxidase C are required to maintain the functional nonplanarity of the heme
- PMID: 12668462
- PMCID: PMC1302820
- DOI: 10.1016/S0006-3495(03)75059-0
The endogenous calcium ions of horseradish peroxidase C are required to maintain the functional nonplanarity of the heme
Abstract
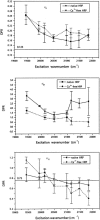
Horseradish peroxidase C (HRPC) binds 2 mol calcium per mol of enzyme with binding sites located distal and proximal to the heme group. The effect of calcium depletion on the conformation of the heme was investigated by combining polarized resonance Raman dispersion spectroscopy with normal coordinate structural decomposition analysis of the hemes extracted from models of Ca(2+)-bound and Ca(2+)-depleted HRPC generated and equilibrated using molecular dynamics simulations. Results show that calcium removal causes reorientation of heme pocket residues. We propose that these rearrangements significantly affect both the in-plane and out-of-plane deformations of the heme. Analysis of the experimental depolarization ratios are clearly consistent with increased B(1g)- and B(2g)-type distortions in the Ca(2+)-depleted species while the normal coordinate structural decomposition results are indicative of increased planarity for the heme of Ca(2+)-depleted HRPC and of significant changes in the relative contributions of three of the six lowest frequency deformations. Most noteworthy is the decrease of the strong saddling deformation that is typical of all peroxidases, and an increase in ruffling. Our results confirm previous work proposing that calcium is required to maintain the structural integrity of the heme in that we show that the preferred geometry for catalysis is lost upon calcium depletion.
Figures






Similar articles
-
The charge transfer band in horseradish peroxidase correlates with heme in-plane distortions induced by calcium removal.Biopolymers. 2004 May-Jun 5;74(1-2):41-5. doi: 10.1002/bip.20040. Biopolymers. 2004. PMID: 15137091
-
Normal mode analysis of the horseradish peroxidase collective motions: correlation with spectroscopically observed heme distortions.Biopolymers. 2006 Jul;82(4):425-9. doi: 10.1002/bip.20463. Biopolymers. 2006. PMID: 16453307
-
Resonance Raman spectroscopy study of change of iron spin state in horseradish peroxidase C induced by removal of calcium.Biopolymers. 2003;72(4):241-8. doi: 10.1002/bip.10417. Biopolymers. 2003. PMID: 12833478
-
Horseradish peroxidase: a modern view of a classic enzyme.Phytochemistry. 2004 Feb;65(3):249-59. doi: 10.1016/j.phytochem.2003.10.022. Phytochemistry. 2004. PMID: 14751298 Review.
-
Horseradish peroxidase: modulation of properties by chemical modification of protein and heme.Biochemistry (Mosc). 2011 Dec;76(13):1391-401. doi: 10.1134/S0006297911130037. Biochemistry (Mosc). 2011. PMID: 22339595 Review.
Cited by
-
Proximity Staining Using Enzymatic Protein Tagging in Diplomonads.mSphere. 2019 Mar 20;4(2):e00153-19. doi: 10.1128/mSphereDirect.00153-19. mSphere. 2019. PMID: 30894436 Free PMC article.
-
Predicting the functionally distinct residues in the heme, cation, and substrate-binding sites of peroxidase from stress-tolerant mangrove specie, Avicennia marina.Cell Stress Chaperones. 2011 Nov;16(6):585-605. doi: 10.1007/s12192-011-0269-3. Epub 2011 Jun 10. Cell Stress Chaperones. 2011. PMID: 21660646 Free PMC article.
-
Structural stabilization and functional improvement of horseradish peroxidase upon modification of accessible lysines: experiments and simulation.Biophys J. 2007 Feb 15;92(4):1192-203. doi: 10.1529/biophysj.106.092858. Epub 2006 Nov 17. Biophys J. 2007. PMID: 17114227 Free PMC article.
-
The tightly bound calcium of MauG is required for tryptophan tryptophylquinone cofactor biosynthesis.Biochemistry. 2011 Jan 11;50(1):144-50. doi: 10.1021/bi101819m. Epub 2010 Dec 13. Biochemistry. 2011. PMID: 21128656 Free PMC article.
-
Production strategies for active heme-containing peroxidases from E. coli inclusion bodies - a review.Biotechnol Rep (Amst). 2016 Mar 24;10:75-83. doi: 10.1016/j.btre.2016.03.005. eCollection 2016 Jun. Biotechnol Rep (Amst). 2016. PMID: 28352527 Free PMC article. Review.
References
-
- Barber, K. R., M. J. Rodriguez Maranon, G. S. Shaw, and R. B. Van Huystee. 1995. Structural influence of calcium on the heme cavity of cationic peanut peroxidase as determined by 1-H-NMR spectroscopy. Eur. J. Biochem. 232:825–833. - PubMed
-
- Bernstein, F. C., T. F. Koetzle, G. J. B. Williams, E. F. Meyer, Jr., M. D. Brice, J. R. Rodgers, O. Kennard, T. Shimanouchi, and M. Tasumi. 1977. The Protein Data Bank: a computer-based archival file for macromolecular structures. Eur. J. Biochem. 80:319–324. - PubMed
-
- Brooks, B. R., R. E. Bruccoleri, B. D. Olafson, D. J. States, S. Swaminathan, and M. Karplus. 1983. CHARMM: a program for macromolecular energy, minimization and dynamics calculations. J. Comp. Chem. 4:187–217.
-
- Chattopadhyay, K., and S. Mazumdar. 2000. Structural and conformational stability of horseradish peroxidase: effect of temperature and pH. Biochemistry. 39:263–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

