Extra spike formation in sensory neurons and the disruption of afferent spike patterning
- PMID: 12668478
- PMCID: PMC1302836
- DOI: 10.1016/S0006-3495(03)75075-9
Extra spike formation in sensory neurons and the disruption of afferent spike patterning
Abstract
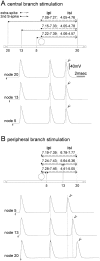
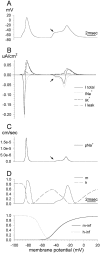
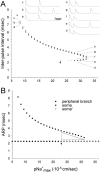
The peculiar pseudounipolar geometry of primary sensory neurons can lead to ectopic generation of "extra spikes" in the region of the dorsal root ganglion potentially disrupting the fidelity of afferent signaling. We have used an explicit model of myelinated vertebrate sensory neurons to investigate the location and mechanism of extra spike formation, and its consequences for distortion of afferent impulse patterning. Extra spikes originate in the initial segment axon under conditions in which the soma spike becomes delayed and broadened. The broadened soma spike then re-excites membrane it has just passed over, initiating an extra spike which propagates outwards into the main conducting axon. Extra spike formation depends on cell geometry, electrical excitability, and the recent history of impulse activity. Extra spikes add to the impulse barrage traveling toward the spinal cord, but they also travel antidromically in the peripheral nerve colliding with and occluding normal orthodromic spikes. As a result there is no net increase in afferent spike number. However, extra spikes render firing more staccato by increasing the number of short and long interspike intervals in the train at the expense of intermediate intervals. There may also be more complex changes in the pattern of afferent spike trains, and hence in afferent signaling.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

