uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion
- PMID: 12668656
- PMCID: PMC2172772
- DOI: 10.1083/jcb.200211091
uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion
Abstract
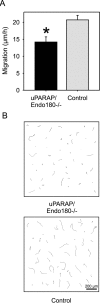
The uptake and lysosomal degradation of collagen by fibroblasts constitute a major pathway in the turnover of connective tissue. However, the molecular mechanisms governing this pathway are poorly understood. Here, we show that the urokinase plasminogen activator receptor-associated protein (uPARAP)/Endo180, a novel mesenchymally expressed member of the macrophage mannose receptor family of endocytic receptors, is a key player in this process. Fibroblasts from mice with a targeted deletion in the uPARAP/Endo180 gene displayed a near to complete abrogation of collagen endocytosis. Furthermore, these cells had diminished initial adhesion to a range of different collagens, as well as impaired migration on fibrillar collagen. These studies identify a central function of uPARAP/Endo180 in cellular collagen interactions.
Figures


References
-
- Ancian, P., G. Lambeau, and M. Lazdunski. 1995. Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry. 34:13146–13151. - PubMed
-
- Barmina, O.Y., H.W. Walling, G.J. Fiacco, J.M. Freije, C. Lopez-Otin, J.J. Jeffrey, and N.C. Partridge. 1999. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J. Biol. Chem. 274:30087–30093. - PubMed
-
- Behrendt, N., E. Rønne, and K. Danø. 1996. Domain interplay in the urokinase receptor. Requirement for the third domain in high affinity ligand binding and demonstration of ligand contact sites in distinct receptor domains. J. Biol. Chem. 271:22885–22894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

