Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth
- PMID: 12668767
- PMCID: PMC153603
- DOI: 10.1073/pnas.0835895100
Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth
Abstract
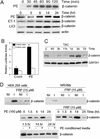
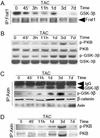
beta-Catenin is a transcriptional activator that regulates embryonic development as part of the Wnt pathway and also plays a role in tumorigenesis. The mechanisms leading to Wnt-induced stabilization of beta-catenin, which results in its translocation to the nucleus and activation of transcription, have been an area of intense interest. However, it is not clear whether stimuli other than Wnts can lead to important stabilization of beta-catenin and, if so, what factors mediate that stabilization and what biologic processes might be regulated. Herein we report that beta-catenin is stabilized in cardiomyocytes after these cells have been exposed to hypertrophic stimuli in culture or in vivo. The mechanism by which beta-catenin is stabilized is distinctly different from that used by Wnt signaling. Although, as with Wnt signaling, inhibition of glycogen synthase kinase-3 remains central to hypertrophic stimulus-induced stabilization of beta-catenin, the mechanism by which this occurs involves the recruitment of activated PKB to the beta-catenin-degradation complex. PKB stabilizes the complex and phosphorylates glycogen synthase kinase-3 within the complex, inhibiting its activity directed at beta-catenin. Finally, we demonstrate via adenoviral gene transfer that beta-catenin is both sufficient to induce growth in cardiomyocytes in culture and in vivo and necessary for hypertrophic stimulus-induced growth. Thus, in these terminally differentiated cells, beta-catenin is stabilized by hypertrophic stimuli acting via heterotrimeric G protein-coupled receptors. The stabilization occurs via a unique Wnt-independent mechanism and results in cellular growth.
Figures


Similar articles
-
Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling.Circ Res. 2005 Jul 22;97(2):144-51. doi: 10.1161/01.RES.0000175241.92285.f8. Epub 2005 Jun 30. Circ Res. 2005. PMID: 15994435
-
The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt. beta-catenin signaling cascade.J Biol Chem. 2004 Apr 9;279(15):14879-88. doi: 10.1074/jbc.M306421200. Epub 2004 Jan 27. J Biol Chem. 2004. PMID: 14747478
-
Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling.Mol Cell Biol. 2003 Feb;23(4):1379-89. doi: 10.1128/MCB.23.4.1379-1389.2003. Mol Cell Biol. 2003. PMID: 12556497 Free PMC article.
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
-
Signaling through beta-catenin and Lef/Tcf.Cell Mol Life Sci. 1999 Oct 30;56(5-6):523-37. doi: 10.1007/s000180050449. Cell Mol Life Sci. 1999. PMID: 11212302 Free PMC article. Review.
Cited by
-
Signal transduction in cancer.Cold Spring Harb Perspect Med. 2015 Apr 1;5(4):a006098. doi: 10.1101/cshperspect.a006098. Cold Spring Harb Perspect Med. 2015. PMID: 25833940 Free PMC article. Review.
-
Eosinophil cationic protein enhances stabilization of β-catenin during cardiomyocyte differentiation in P19CL6 embryonal carcinoma cells.Mol Biol Rep. 2013 Apr;40(4):3165-71. doi: 10.1007/s11033-012-2390-5. Epub 2012 Dec 28. Mol Biol Rep. 2013. PMID: 23271121
-
Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis.J Biol Chem. 2010 May 7;285(19):14756-63. doi: 10.1074/jbc.M110.102970. Epub 2010 Mar 8. J Biol Chem. 2010. PMID: 20212039 Free PMC article.
-
Neuroprotective actions of ghrelin and growth hormone secretagogues.Front Mol Neurosci. 2011 Sep 28;4:23. doi: 10.3389/fnmol.2011.00023. eCollection 2011. Front Mol Neurosci. 2011. PMID: 21994488 Free PMC article.
-
A therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome.Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2541-50. doi: 10.1152/ajpheart.01052.2008. Epub 2008 Oct 31. Am J Physiol Heart Circ Physiol. 2008. PMID: 18978187 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

