Transcriptional oscillation of lunatic fringe is essential for somitogenesis
- PMID: 12670869
- PMCID: PMC196028
- DOI: 10.1101/gad.250603
Transcriptional oscillation of lunatic fringe is essential for somitogenesis
Abstract
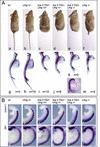
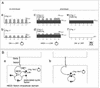
A molecular oscillator that controls the expression of cyclic genes such as lunatic fringe (Lfng) in the presomitic mesoderm has been shown to be coupled with somite formation in vertebrate embryos. To address the functional significance of oscillating Lfng expression, we have generated transgenic mice expressing Lfng constitutively in the presomitic mesoderm in addition to the intrinsic cyclic Lfng activity. These transgenic lines displayed defects of somite patterning and vertebral organization that were very similar to those of Lfng null mutants. Furthermore, constitutive expression of exogenous Lfng did not compensate for the complete loss of cyclic endogenous Lfng activity. Noncyclic exogenous Lfng expression did not abolish cyclic expression of endogenous Lfng in the posterior presomitic mesoderm (psm) but affected its expression pattern in the anterior psm. Similarly, dynamic expression of Hes7 was not abolished but abnormal expression patterns were obtained. Our data are consistent with a model in which alternations of Lfng activity between ON and OFF states in the presomitic mesoderm prior to somite segmentation are critical for proper somite patterning, and suggest that Notch signaling might not be the only determinant of cyclic gene expression in the presomitic mesoderm of mouse embryos.
Figures




References
-
- Aulehla A, Johnson RL. Dynamic expression of lunatic fringe suggests a link between notch signaling and an autonomous cellular oscillator driving somite segmentation. Dev Biol. 1999;207:49–61. - PubMed
-
- Aulehla, A., Wehrle, C., Kemler, R., Mallo, M., Gossler, A., Kanzler, B., and Herrmann, B.G. 2003. Wnt acts upstream of Notch in the segmentation clock controlling somitogenesis. Dev. Cell (In press). - PubMed
-
- Beckers J, Caron A, Hrabe de Angelis M, Hans S, Campos-Ortega JA, Gossler A. Distinct regulatory elements direct Delta1 expression in the nervous system and paraxial mesoderm of transgenic mice. Mech Dev. 2000;95:23–34. - PubMed
-
- Bessho Y, Miyoshi G, Sakata R, Kageyama R. Hes7: A bHLH-type repressor gene regulated by Notch and expressed in the presomitic mesoderm. Genes Cells. 2001a;6:175–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
