Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis
- PMID: 12670969
- PMCID: PMC152624
- DOI: 10.1128/JB.185.8.2457-2464.2003
Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis
Abstract
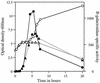
The rates of germination of Bacillus subtilis spores with L-alanine were increased markedly, in particular at low L-alanine concentrations, by overexpression of the tricistronic gerA operon that encodes the spore's germinant receptor for L-alanine but not by overexpression of gerA operon homologs encoding receptors for other germinants. However, spores with elevated levels of the GerA proteins did not germinate more rapidly in a mixture of asparagine, glucose, fructose, and K(+) (AGFK), a germinant combination that requires the participation of at least the germinant receptors encoded by the tricistronic gerB and gerK operons. Overexpression of the gerB or gerK operon or both the gerB and gerK operons also did not stimulate spore germination in AGFK. Overexpression of a mutant gerB operon, termed gerB*, that encodes a receptor allowing spore germination in response to either D-alanine or L-asparagine also caused faster spore germination with these germinants, again with the largest enhancement of spore germination rates at lower germinant concentrations. However, the magnitudes of the increases in the germination rates with D-alanine or L-asparagine in spores overexpressing gerB* were well below the increases in the spore's levels of the GerBA protein. Germination of gerB* spores with D-alanine or L-asparagine did not require participation of the products of the gerK operon, but germination with these agents was decreased markedly in spores also overexpressing gerA. These findings suggest that (i) increases in the levels of germinant receptors that respond to single germinants can increase spore germination rates significantly; (ii) there is some maximum rate of spore germination above which stimulation of GerA operon receptors alone will not further increase the rate of spore germination, as action of some protein other than the germinant receptors can become rate limiting; (iii) while previous work has shown that the wild-type GerB and GerK receptors interact in some fashion to cause spore germination in AGFK, there also appears to be an additional component required for AGFK-triggered spore germination; (iv) activation of the GerB receptor with D-alanine or L-asparagine can trigger spore germination independently of the GerK receptor; and (v) it is likely that the different germinant receptors interact directly and/or compete with each other for some additional component needed for initiation of spore germination. We also found that very high levels of overexpression of the gerA or gerK operon (but not the gerB or gerB* operon) in the forespore blocked sporulation shortly after the engulfment stage, although sporulation appeared normal with the lower levels of gerA or gerK overexpression that were used to generate spores for analysis of rates of germination.
Figures

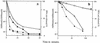
References
-
- Auer, M., M. J. Kim, A. Villa, J. Song, X. D. Wang, and D. N. Wang. 2001. High-yield expression and functional analysis of Escherichia coli glycerol-3-phosphate transporter. Biochemistry 40:6628-6635. - PubMed
-
- Barlass, P. J., C. W. Houston, M. O. Clements, and A. Moir. 2002. Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089-2095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

