Regulation of the alternative sigma factor sigma(E) during initiation, adaptation, and shutoff of the extracytoplasmic heat shock response in Escherichia coli
- PMID: 12670975
- PMCID: PMC152616
- DOI: 10.1128/JB.185.8.2512-2519.2003
Regulation of the alternative sigma factor sigma(E) during initiation, adaptation, and shutoff of the extracytoplasmic heat shock response in Escherichia coli
Abstract
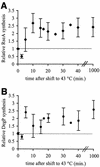
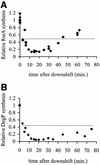
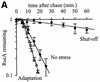
The alternative sigma factor sigma(E) is activated in response to stress in the extracytoplasmic compartment of Escherichia coli. Here we show that sigma(E) activity increases upon initiation of the stress response by a shift to an elevated temperature (43 degrees C) and remains at that level for the duration of the stress. When the stress is removed by a temperature downshift, sigma(E) activity is strongly repressed and then slowly returns to levels seen in unstressed cells. We provide evidence that information about the state of the cell envelope is communicated to sigma(E) primarily through the regulated proteolysis of the inner membrane anti-sigma factor RseA, as the degradation rate of RseA is correlated with the changes in sigma(E) activity throughout the stress response. However, the relationship between sigma(E) activity and the rate of degradation of RseA is complex, indicating that other factors may cooperate with RseA and serve to fine-tune the response.
Figures





References
-
- Alba, B. M., H. J. Zhong, J. C. Pelayo, and C. A. Gross. 2001. degS (hhoB) is an essential Escherichia coli gene whose indispensable function is to provide sigmaE activity. Mol. Microbiol. 40:1323-1333. - PubMed
-
- Becker, J., and E. A. Craig. 1994. Heat-shock proteins as molecular chaperones. Eur. J. Biochem. 219:11-23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

