Pseudomonas syringae exchangeable effector loci: sequence diversity in representative pathovars and virulence function in P. syringae pv. syringae B728a
- PMID: 12670984
- PMCID: PMC152607
- DOI: 10.1128/JB.185.8.2592-2602.2003
Pseudomonas syringae exchangeable effector loci: sequence diversity in representative pathovars and virulence function in P. syringae pv. syringae B728a
Abstract
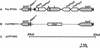
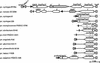
Pseudomonas syringae is a plant pathogen whose pathogenicity and host specificity are thought to be determined by Hop/Avr effector proteins injected into plant cells by a type III secretion system. P. syringae pv. syringae B728a, which causes brown spot of bean, is a particularly well-studied strain. The type III secretion system in P. syringae is encoded by hrp (hypersensitive response and pathogenicity) and hrc (hrp conserved) genes, which are clustered in a pathogenicity island with a tripartite structure such that the hrp/hrc genes are flanked by a conserved effector locus and an exchangeable effector locus (EEL). The EELs of P. syringae pv. syringae B728a, P. syringae strain 61, and P. syringae pv. tomato DC3000 differ in size and effector gene composition; the EEL of P. syringae pv. syringae B728a is the largest and most complex. The three putative effector proteins encoded by the P. syringae pv. syringae B728a EEL--HopPsyC, HopPsyE, and HopPsyV--were demonstrated to be secreted in an Hrp-dependent manner in culture. Heterologous expression of hopPsyC, hopPsyE, and hopPsyV in P. syringae pv. tabaci induced the hypersensitive response in tobacco leaves, demonstrating avirulence activity in a nonhost plant. Deletion of the P. syringae pv. syringae B728a EEL strongly reduced virulence in host bean leaves. EELs from nine additional strains representing nine P. syringae pathovars were isolated and sequenced. Homologs of avrPphE (e.g., hopPsyE) and hopPsyA were particularly common. Comparative analyses of these effector genes and hrpK (which flanks the EEL) suggest that the EEL effector genes were acquired by horizontal transfer after the acquisition of the hrp/hrc gene cluster but before the divergence of modern pathovars and that some EELs underwent transpositions yielding effector exchanges or point mutations producing effector pseudogenes after their acquisition.
Figures


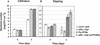

References
-
- Alfano, J. R., D. W. Bauer, T. M. Milos, and A. Collmer. 1996. Analysis of the role of the Pseudomonas syringae pv. syringae HrpZ harpin in elicitation of the hypersensitive response in tobacco using functionally non-polar hrpZ deletion mutations, truncated HrpZ fragments, and hrmA mutations. Mol. Microbiol. 19:715-728. - PubMed
-
- Alfano, J. R., A. O. Charkowski, W.-L. Deng, J. L. Badel, T. Petnicki-Ocwieja, K. van Dijk, and A. Collmer. 2000. The Pseudomonas syringae Hrp pathogenicity island has a tripartite mosaic structure composed of a cluster of type III secretion genes bounded by exchangeable effector and conserved effector loci that contribute to parasitic fitness and pathogenicity in plants. Proc. Natl. Acad. Sci. USA 97:4856-4861. - PMC - PubMed
-
- Alfano, J. R., H. S. Kim, T. P. Delaney, and A. Collmer. 1997. Evidence that the Pseudomonas syringae pv. syringae hrp-linked hrmA gene encodes an Avr-like protein that acts in an hrp-dependent manner within tobacco cells. Mol. Plant-Microbe Interact. 10:580-588. - PubMed
-
- Bergman, T., K. Erickson, E. Galyov, C. Persson, and H. Wolf-Watz. 1994. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J. Bacteriol. 176:2619-2626. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

