WNK kinases regulate thiazide-sensitive Na-Cl cotransport
- PMID: 12671053
- PMCID: PMC152590
- DOI: 10.1172/JCI17443
WNK kinases regulate thiazide-sensitive Na-Cl cotransport
Abstract
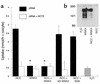
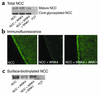
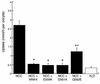
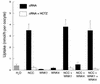
Pseudohypoaldosteronism type II (PHAII) is an autosomal dominant disorder of hyperkalemia and hypertension. Mutations in two members of the WNK kinase family, WNK1 and WNK4, cause the disease. WNK1 mutations are believed to increase WNK1 expression; the effect of WNK4 mutations remains unknown. The clinical phenotype of PHAII is opposite to Gitelman syndrome, a disease caused by dysfunction of the thiazide-sensitive Na-Cl cotransporter. We tested the hypothesis that WNK kinases regulate the mammalian thiazide-sensitive Na-Cl cotransporter (NCC). Mouse WNK4 was cloned and expressed in Xenopus oocytes with or without NCC. Coexpression with WNK4 suppressed NCC activity by more than 85%. This effect did not result from defects in NCC synthesis or processing, but was associated with an 85% reduction in NCC abundance at the plasma membrane. Unlike WNK4, WNK1 did not affect NCC activity directly. WNK1, however, completely prevented WNK4 inhibition of NCC. Some WNK4 mutations that cause PHAII retained NCC-inhibiting activity, but the Q562E WNK4 demonstrated diminished activity, suggesting that some PHAII mutations lead to loss of NCC inhibition. Gain-of-function WNK1 mutations would be expected to inhibit WNK4 activity, thereby activating NCC, contributing to the PHAII phenotype. Together, these results identify WNK kinases as a previously unrecognized sodium regulatory pathway of the distal nephron. This pathway likely contributes to normal and pathological blood pressure homeostasis.
Figures
Comment in
-
Negative regulators of sodium transport in the kidney: key factors in understanding salt-sensitive hypertension?J Clin Invest. 2003 Apr;111(7):947-50. doi: 10.1172/JCI18232. J Clin Invest. 2003. PMID: 12671041 Free PMC article. Review. No abstract available.
References
-
- Schambelan M, Sebastian A, Rector FC., Jr Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int. 1981;19:716–727. - PubMed
-
- Mayan H, et al. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J. Clin. Endocrinol. Metab. 2002;87:3248–3254. - PubMed
-
- Simon DB, et al. Examination of the thiazide-sensitive Na-Cl cotransporter as a candidate gene in Gordon’s syndrome. J. Am. Soc. Nephrol. 1995;6:632. (Abstr.)
-
- Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. - PubMed
-
- Xu B, et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275:16795–16801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

