The endomembrane requirement for cell surface repair
- PMID: 12672953
- PMCID: PMC153600
- DOI: 10.1073/pnas.0736739100
The endomembrane requirement for cell surface repair
Abstract
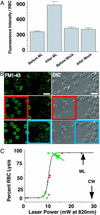
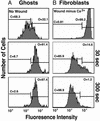
The capacity to reseal a plasma membrane disruption rapidly is required for cell survival in many physiological environments. Intracellular membrane (endomembrane) is thought to play a central role in the rapid resealing response. We here directly compare the resealing response of a cell that lacks endomembrane, the red blood cell, with that of several nucleated cells possessing an abundant endomembrane compartment. RBC membrane disruptions inflicted by a mode-locked Ti:sapphire laser, even those initially smaller than hemoglobin, failed to reseal rapidly. By contrast, much larger laser-induced disruptions made in sea urchin eggs, fibroblasts, and neurons exhibited rapid, Ca(2+)-dependent resealing. We conclude that rapid resealing is not mediated by simple physiochemical mechanisms; endomembrane is required.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

