Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation
- PMID: 12672956
- PMCID: PMC153580
- DOI: 10.1073/pnas.0735712100
Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation
Abstract
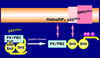
Protein-phosphoinositide interaction participates in targeting proteins to membranes where they function correctly and is often modulated by phosphorylation of lipids. Here we show that protein phosphorylation of p47(phox), a cytoplasmic activator of the microbicidal phagocyte oxidase (phox), elicits interaction of p47(phox) with phosphoinositides. Although the isolated phox homology (PX) domain of p47(phox) can interact directly with phosphoinositides, the lipid-binding activity of this protein is normally suppressed by intramolecular interaction of the PX domain with the C-terminal Src homology 3 (SH3) domain, and hence the wild-type full-length p47(phox) is incapable of binding to the lipids. The W263R substitution in this SH3 domain, abrogating the interaction with the PX domain, leads to a binding of p47(phox) to phosphoinositides. The findings indicate that disruption of the intramolecular interaction renders the PX domain accessible to the lipids. This conformational change is likely induced by phosphorylation of p47(phox), because protein kinase C treatment of the wild-type p47(phox) but not of a mutant protein with the S303304328A substitution culminates in an interaction with phosphoinositides. Furthermore, although the wild-type p47(phox) translocates upon cell stimulation to membranes to activate the oxidase, neither the kinase-insensitive p47(phox) nor lipid-binding-defective proteins, one lacking the PX domain and the other carrying the R90K substitution in this domain, migrates. Thus the protein phosphorylation-driven conformational change of p47(phox) enables its PX domain to bind to phosphoinositides, the interaction of which plays a crucial role in recruitment of p47(phox) from the cytoplasm to membranes and subsequent activation of the phagocyte oxidase.
Figures
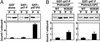

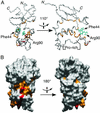

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

