The effects of nicotinic and muscarinic receptor activation on patch-clamped cells in the optic tectum of Rana pipiens
- PMID: 12676145
- PMCID: PMC2265077
- DOI: 10.1016/s0306-4522(02)00768-6
The effects of nicotinic and muscarinic receptor activation on patch-clamped cells in the optic tectum of Rana pipiens
Abstract
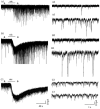
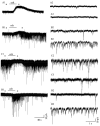
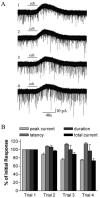
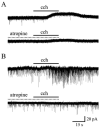
Both nicotinic and muscarinic cholinergic receptors are present in the optic tectum. To begin to understand how the activation of these receptors affects visual activity patterns, we have determined the types of physiological responses induced by their activation. Using tectal brain slices from the leopard frog, we found that application of nicotine (100 microM) evoked long-lasting responses in 60% of patch-clamped tectal cells. Thirty percent of these responses consisted of an increase in spontaneous postsynaptic currents (sPSCs) and had both a glutamatergic and GABAergic component as determined by the use of 6-cyano-7-nitroquinoxaline-2,3-dione (50 microM) and bicuculline (25 microM), respectively. Remaining response types consisted of an inward membrane current (16%) and an increase in sPSCs combined with an inward membrane current (14%). All responses could be elicited in the presence of tetrodotoxin (0.5 microM). Muscarinic receptor-mediated responses, induced by carbachol (100 microM) application after nicotinic receptor desensitization, produced responses in 70% of tectal cells. In contrast to responses elicited by nicotine, carbachol-induced responses could be evoked multiple times without significant decrement. Responses consisted of either an outward current (57%), a decrease in sPSCs (5%) or an increase in sPSCs, with (almost 6%) or without (almost 3%) an outward current. The response elicited by carbachol was not predicted by the response of the cell to nicotine. Our results suggest that nicotinic receptors are found predominantly at presynaptic locations in the optic tectum while muscarinic receptors are most often present at postsynaptic sites. We conclude that both of these receptor types could substantially modulate visual activity by changing either the input to tectal neurons or the level of their response to that input.
Figures

Similar articles
-
Bidirectional modulation of visual plasticity by cholinergic receptor subtypes in the frog optic tectum.Eur J Neurosci. 2003 Mar;17(6):1253-65. doi: 10.1046/j.1460-9568.2003.02557.x. Eur J Neurosci. 2003. PMID: 12670313 Free PMC article.
-
Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus.Eur J Neurosci. 2006 Dec;24(11):3096-108. doi: 10.1111/j.1460-9568.2006.05190.x. Eur J Neurosci. 2006. PMID: 17156371
-
Potentiation of glutamatergic synaptic input to supraoptic neurons by presynaptic nicotinic receptors.Am J Physiol Regul Integr Comp Physiol. 2001 Oct;281(4):R1105-13. doi: 10.1152/ajpregu.2001.281.4.R1105. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11557616
-
Pharmacology, distribution and development of muscarinic acetylcholine receptor subtypes in the optic tectum of Rana pipiens.Neuroscience. 2001;104(1):161-79. doi: 10.1016/s0306-4522(01)00048-3. Neuroscience. 2001. PMID: 11311540 Free PMC article.
-
Silent synapses sit and wait for a better day.Neuron. 2009 Jan 29;61(2):157-9. doi: 10.1016/j.neuron.2009.01.004. Neuron. 2009. PMID: 19186159 Free PMC article. Review.
Cited by
-
Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum.Neuron. 2008 Nov 26;60(4):698-708. doi: 10.1016/j.neuron.2008.09.013. Neuron. 2008. PMID: 19038225 Free PMC article.
-
Stimulus-driven competition in a cholinergic midbrain nucleus.Nat Neurosci. 2010 Jul;13(7):889-95. doi: 10.1038/nn.2573. Epub 2010 Jun 6. Nat Neurosci. 2010. PMID: 20526331 Free PMC article.
-
Bidirectional modulation of visual plasticity by cholinergic receptor subtypes in the frog optic tectum.Eur J Neurosci. 2003 Mar;17(6):1253-65. doi: 10.1046/j.1460-9568.2003.02557.x. Eur J Neurosci. 2003. PMID: 12670313 Free PMC article.
-
Electrophysiological properties of isthmic neurons in frogs revealed by in vitro and in vivo studies.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Apr;196(4):249-62. doi: 10.1007/s00359-010-0511-y. Epub 2010 Feb 24. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 20179943 Free PMC article.
-
Muscarinic inhibition of recurrent glutamatergic excitation in frog tectum column prevents NMDA receptor activation on efferent neuron.Exp Brain Res. 2011 Feb;208(3):323-34. doi: 10.1007/s00221-010-2484-z. Epub 2010 Nov 17. Exp Brain Res. 2011. PMID: 21082312
References
-
- Aubert I, Cecyre D, Gauthier S, Quirion R. Characterization and autoradiographic distribution of [3H]AF-DX 384 binding to putative muscarinic M2 receptors in the rat brain. Eur J Pharmacol. 1992;217:173–184. - PubMed
-
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. - PubMed
-
- Bickford ME, Ramcharan E, Godwin DW, Erisir A, Gnadt J, Sherman SM. Neurotransmitters contained in the subcortical extraretinal inputs to the monkey lateral geniculate nucleus. J Comp Neurol. 2000;424:701–717. - PubMed
-
- Binns KE. The synaptic pharmacology underlying sensory processing in the superior colliculus. Prog Neurobiol. 1999;59:129–159. - PubMed
-
- Binns KE, Salt TE. The functional influence of nicotinic cholinergic receptors on the visual responses of neurones in the superficial superior colliculus. Vis Neurosci. 2000;17:283–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

