Effect of signal compounds and incubation conditions on the culturability of freshwater bacterioplankton
- PMID: 12676673
- PMCID: PMC154826
- DOI: 10.1128/AEM.69.4.1980-1989.2003
Effect of signal compounds and incubation conditions on the culturability of freshwater bacterioplankton
Abstract
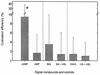
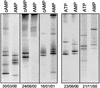
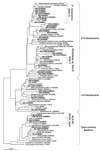
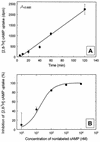
The effect of signal compounds and of different incubation conditions on the culturability (i.e., the fraction of all cells capable of growth) of natural bacterioplankton from the eutrophic lake Zwischenahner Meer was investigated over a period of 20 months. Numbers of growing cells were determined by the most-probable-number technique in liquid media containing low concentrations (10 micro M) of the signal compounds N-(oxohexanoyl)-DL-homoserine lactone, N-(butyryl)-DL-homoserine lactone, cyclic AMP (cAMP), or ATP. cAMP was the most effective signal compound, leading to significantly increased cultivation efficiencies of up to 10% of the total bacterial counts. Microautoradiography with [2,8-(3)H]cAMP, combined with fluorescence in situ hybridization, demonstrated that cAMP was taken up by 18% of all cells. The bacterial cAMP uptake systems had a very low K(m) value of </=1 nM. Analysis of the cultured bacteria by 16S rRNA gene fingerprinting showed that different bacterial phylotypes were recovered in the presence and in the absence of cAMP. Consequently, the addition of cAMP caused a stimulation of otherwise nonculturable bacteria. Phylogenetically different bacteria were also recovered at different temperatures and oxygen partial pressures. Throughout the study period, mainly members of the beta-subclass of the Proteobacteria were cultivated. In addition, some members of the Actinomycetales were enriched. Quantification by culture-independent fluorescence in situ hybridization demonstrated that beta-Proteobacteria and Actinomycetales also dominated the natural bacterioplankton assemblage. Sequence comparison revealed that two members of the Actinomycetales which reached high numbers in the natural bacterioplankton assemblage could actually be enriched by our cultivation approach.
Figures


References
-
- Amann, R. I. 1995. Fluorescently labelled, rRNA-targeted oligonucleotide probes in the study of microbial ecology. Mol. Ecol. 4:543-553.
-
- Ammerman, J. W., and F. Azam. 1981. Dissolved cyclic adenosine monophosphate (cAMP) in the sea and uptake of cAMP by marine bacteria. Mar. Ecol. Prog. Ser. 5:85-89.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

