Overproduction of threonine aldolase circumvents the biosynthetic role of pyruvate decarboxylase in glucose-limited chemostat cultures of Saccharomyces cerevisiae
- PMID: 12676688
- PMCID: PMC154831
- DOI: 10.1128/AEM.69.4.2094-2099.2003
Overproduction of threonine aldolase circumvents the biosynthetic role of pyruvate decarboxylase in glucose-limited chemostat cultures of Saccharomyces cerevisiae
Abstract
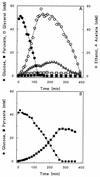
Pyruvate decarboxylase-negative (Pdc(-)) mutants of Saccharomyces cerevisiae require small amounts of ethanol or acetate to sustain aerobic, glucose-limited growth. This nutritional requirement has been proposed to originate from (i) a need for cytosolic acetyl coenzyme A (acetyl-CoA) for lipid and lysine biosynthesis and (ii) an inability to export mitochondrial acetyl-CoA to the cytosol. To test this hypothesis and to eliminate the C(2) requirement of Pdc(-) S. cerevisiae, we attempted to introduce an alternative pathway for the synthesis of cytosolic acetyl-CoA. The addition of L-carnitine to growth media did not restore growth of a Pdc(-) strain on glucose, indicating that the C(2) requirement was not solely due to the inability of S. cerevisiae to synthesize this compound. The S. cerevisiae GLY1 gene encodes threonine aldolase (EC 4.1.2.5), which catalyzes the cleavage of threonine to glycine and acetaldehyde. Overexpression of GLY1 enabled a Pdc(-) strain to grow under conditions of carbon limitation in chemostat cultures on glucose as the sole carbon source, indicating that acetaldehyde formed by threonine aldolase served as a precursor for the synthesis of cytosolic acetyl-CoA. Fractionation studies revealed a cytosolic localization of threonine aldolase. The absence of glycine in these cultures indicates that all glycine produced by threonine aldolase was either dissimilated or assimilated. These results confirm the involvement of pyruvate decarboxylase in cytosolic acetyl-CoA synthesis. The Pdc(-) GLY1 overexpressing strain was still glucose sensitive with respect to growth in batch cultivations. Like any other Pdc(-) strain, it failed to grow on excess glucose in batch cultures and excreted pyruvate when transferred from glucose limitation to glucose excess.
Figures

Similar articles
-
Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae.FEMS Microbiol Lett. 1999 May 1;174(1):73-9. doi: 10.1111/j.1574-6968.1999.tb13551.x. FEMS Microbiol Lett. 1999. PMID: 10234824
-
Directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C2-independent, glucose-tolerant, and pyruvate-hyperproducing yeast.Appl Environ Microbiol. 2004 Jan;70(1):159-66. doi: 10.1128/AEM.70.1.159-166.2004. Appl Environ Microbiol. 2004. PMID: 14711638 Free PMC article.
-
Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose.Yeast. 1996 Mar 15;12(3):247-57. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C247::AID-YEA911%3E3.0.CO;2-I. Yeast. 1996. PMID: 8904337
-
Engineering cytosolic acetyl-coenzyme A supply in Saccharomyces cerevisiae: Pathway stoichiometry, free-energy conservation and redox-cofactor balancing.Metab Eng. 2016 Jul;36:99-115. doi: 10.1016/j.ymben.2016.03.006. Epub 2016 Mar 23. Metab Eng. 2016. PMID: 27016336 Review.
-
Rewiring yeast metabolism to synthesize products beyond ethanol.Curr Opin Chem Biol. 2020 Dec;59:182-192. doi: 10.1016/j.cbpa.2020.08.005. Epub 2020 Oct 5. Curr Opin Chem Biol. 2020. PMID: 33032255 Free PMC article. Review.
Cited by
-
Homofermentative lactate production cannot sustain anaerobic growth of engineered Saccharomyces cerevisiae: possible consequence of energy-dependent lactate export.Appl Environ Microbiol. 2004 May;70(5):2898-905. doi: 10.1128/AEM.70.5.2898-2905.2004. Appl Environ Microbiol. 2004. PMID: 15128549 Free PMC article.
-
Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia.Plant Cell. 2005 Aug;17(8):2355-68. doi: 10.1105/tpc.105.033290. Epub 2005 Jul 1. Plant Cell. 2005. PMID: 15994907 Free PMC article.
-
Improving isobutanol production with the yeast Saccharomyces cerevisiae by successively blocking competing metabolic pathways as well as ethanol and glycerol formation.Biotechnol Biofuels. 2019 Jul 2;12:173. doi: 10.1186/s13068-019-1486-8. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31303893 Free PMC article.
-
Interkingdom Comparison of Threonine Metabolism for Stem Cell Maintenance in Plants and Animals.Front Cell Dev Biol. 2021 Sep 7;9:672545. doi: 10.3389/fcell.2021.672545. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34557481 Free PMC article. Review.
-
Genome-scale model-driven strain design for dicarboxylic acid production in Yarrowia lipolytica.BMC Syst Biol. 2018 Mar 19;12(Suppl 2):12. doi: 10.1186/s12918-018-0542-5. BMC Syst Biol. 2018. PMID: 29560822 Free PMC article.
References
-
- Atomi, H., M. Ueda, J. Suzuki, Y. Kamada, and A. Tanaka. 1993. Presence of carnitine acyltransferase in peroxisomes and in mitochondria of oleic acid-grown Saccharomyces cerevisiae. FEMS Microbiol. Lett. 112:31-34. - PubMed
-
- Bianchi, M. M., L. Tizzani, M. Destruelle, L. Frontali, and M. Wésolowski-Louvel. 1996. The “petite-negative” yeast Kluyveromyces lactis has a single gene expressing pyruvate decarboxylase activity. Mol. Microbiol. 19:27-36. - PubMed
-
- Diderich, J. A., L. M. Raamsdonk, A. Kuiper, A. L. Kruckeberg, M. J. Teixeira de Mattos, and K. van Dam. 2002. Effects of a hexokinase II deletion on the dynamics of glycolysis in continuous cultures of Saccaromyces cerevisiae. FEMS Yeast Res. 2:165-172. - PubMed
-
- Douma, A. C., M. Veenhuis, W. de Koning, M. Evers, and W. Harder. 1985. Dihydroxyacetone synthase is localized in the peroxisomal matrix of methanol-grown Hansenula polymorpha. Arch. Microbiol. 143:237-243.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

