Bacterioplankton community shifts in an arctic lake correlate with seasonal changes in organic matter source
- PMID: 12676708
- PMCID: PMC154827
- DOI: 10.1128/AEM.69.4.2253-2268.2003
Bacterioplankton community shifts in an arctic lake correlate with seasonal changes in organic matter source
Abstract
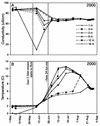
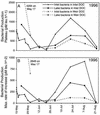
Seasonal shifts in bacterioplankton community composition in Toolik Lake, a tundra lake on the North Slope of Alaska, were related to shifts in the source (terrestrial versus phytoplankton) and lability of dissolved organic matter (DOM). A shift in community composition, measured by denaturing gradient gel electrophoresis (DGGE) of 16S rRNA genes, occurred at 4 degrees C in near-surface waters beneath seasonal ice and snow cover in spring. This shift was associated with an annual peak in bacterial productivity ([(14)C]leucine incorporation) driven by the large influx of labile terrestrial DOM associated with snow meltwater. A second shift occurred after the flux of terrestrial DOM had ended in early summer as ice left the lake and as the phytoplankton community developed. Bacterioplankton communities were composed of persistent populations present throughout the year and transient populations that appeared and disappeared. Most of the transient populations could be divided into those that were advected into the lake with terrestrial DOM in spring and those that grew up from low concentrations during the development of the phytoplankton community in early summer. Sequencing of DNA in DGGE bands demonstrated that most bands represented single ribotypes and that matching bands from different samples represented identical ribotypes. Bacteria were identified as members of globally distributed freshwater phylogenetic clusters within the alpha- and beta-Proteobacteria, the Cytophaga-Flavobacteria-Bacteroides group, and the ACTINOBACTERIA:
Figures









References
-
- Bahr, M., J. E. Hobbie, and M. L. Sogin. 1996. Bacterial diversity in an Arctic lake: a freshwater SAR11 cluster. Aquat. Microb. Ecol. 11:271-277.
-
- Bergström A.-K., and M. Jansson. 2000. Bacterioplankton production in humic Lake Örträsket in relation to input of bacterial cells and input of allochthonous organic carbon. Microb. Ecol. 39:101-115. - PubMed
-
- Castberg, T., A. Larsen, R. A. Sandaa, C. P. D. Brussaard, J. K. Egge, M. Heldal, R. Thyrhaug, E. J. van Hannen, and G. Bratbak. 2001. Microbial population dynamics and diversity during a bloom of marine coccolithophorid Emiliania huxleyi (Haptophyta). Mar. Ecol. Prog. Ser. 221:39-46.
-
- Cole, J. J., S. Findlay, and M. L. Pace. 1988. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar. Ecol. Prog. Ser. 43:1-10.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

