Disinfection of water containing natural organic matter by using ozone-initiated radical reactions
- PMID: 12676711
- PMCID: PMC154773
- DOI: 10.1128/AEM.69.4.2284-2291.2003
Disinfection of water containing natural organic matter by using ozone-initiated radical reactions
Abstract
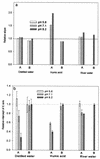
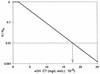
Ozone is widely used to disinfect drinking water and wastewater due to its strong biocidal oxidizing properties. Recently, it was reported that hydroxyl radicals ((.)OH), resulting from ozone decomposition, play a significant role in microbial inactivation when Bacillus subtilis endospores were used as the test microorganisms in pH controlled distilled water. However, it is not yet known how natural organic matter (NOM), which is ubiquitous in sources of drinking water, affects this process of disinfection by ozone-initiated radical reactions. Two types of water matrix were considered for this study. One is water containing humic acid, which is commercially available. The other is water from the Han River. This study reported that hydroxyl radicals, initiated by the ozone chain reaction, were significantly effective at B. subtilis endospore inactivation in water containing NOM, as well as in pH-controlled distilled water. The type of NOM and the pH have a considerable effect on the percentage of disinfection by hydroxyl radicals, which ranged from 20 to 50%. In addition, the theoretical T value of hydroxyl radicals for 2-log B. subtilis removal was estimated to be about 2.4 x 10(4) times smaller than that of ozone, assuming that there is no synergistic activity between ozone and hydroxyl radicals.
Figures



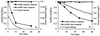

Similar articles
-
Inactivation of Bacillus subtilis spores during ozonation in water treatment plant: influence of pre-treatment and consequences for positioning of the ozonation step.Chemosphere. 2007 Oct;69(5):675-81. doi: 10.1016/j.chemosphere.2007.05.045. Epub 2007 Jul 2. Chemosphere. 2007. PMID: 17604815
-
Inactivation of Bacillus subtilis spores with ozone and monochloramine.Water Res. 2003 Feb;37(4):833-44. doi: 10.1016/s0043-1354(02)00381-0. Water Res. 2003. PMID: 12531265
-
Response surface methodology as a tool for modeling and optimization of Bacillus subtilis spores inactivation by UV/ nano-Fe0 process for safe water production.Food Chem Toxicol. 2018 Apr;114:334-345. doi: 10.1016/j.fct.2018.02.045. Epub 2018 Feb 23. Food Chem Toxicol. 2018. PMID: 29481893
-
Ozonation of drinking water: part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine.Water Res. 2003 Apr;37(7):1469-87. doi: 10.1016/S0043-1354(02)00458-X. Water Res. 2003. PMID: 12600375 Review.
-
Removal of natural organic matter (NOM) from water by ion exchange - A review.Chemosphere. 2018 Feb;192:90-104. doi: 10.1016/j.chemosphere.2017.10.101. Epub 2017 Oct 19. Chemosphere. 2018. PMID: 29100126 Review.
Cited by
-
Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection.Appl Environ Microbiol. 2005 Jan;71(1):270-5. doi: 10.1128/AEM.71.1.270-275.2005. Appl Environ Microbiol. 2005. PMID: 15640197 Free PMC article.
-
Disinfection by Ozone Microbubbles Can Cause Morphological Change of Fusarium oxysporum f. sp. melonis Spores.Plant Pathol J. 2018 Aug;34(4):335-340. doi: 10.5423/PPJ.NT.11.2017.0234. Epub 2018 Aug 1. Plant Pathol J. 2018. PMID: 30140187 Free PMC article.
-
Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review.Foods. 2021 Mar 12;10(3):605. doi: 10.3390/foods10030605. Foods. 2021. PMID: 33809297 Free PMC article. Review.
-
Ozone Decontamination of Medical and Nonmedical Devices: An Assessment of Design and Implementation Considerations.Ind Eng Chem Res. 2023 Mar 1;62(10):4191-4209. doi: 10.1021/acs.iecr.2c03754. eCollection 2023 Mar 15. Ind Eng Chem Res. 2023. PMID: 36943762 Free PMC article. Review.
-
Antimicrobial efficacy of aqueous ozone in combination with short chain fatty acid buffers.Infect Prev Pract. 2019 Dec 16;2(1):100032. doi: 10.1016/j.infpip.2019.100032. eCollection 2020 Mar. Infect Prev Pract. 2019. PMID: 34368688 Free PMC article.
References
-
- Bancroft, K., P. Chrostowski, R. L. Wright, and I. H. Suffet. 1984. Ozonation and oxidation competition values: relationship to disinfection and microorganisms regrowth. Water Res. 4:473-478.
-
- Cho, M., H. Chung, and J. Yoon. 2002. Effect of pH and importance of ozone initiated radical reactions in inactivating Bacillus subtilis spore. Ozone Sci. Eng. 24:145-150.
-
- Dahi, E. 1976. Physicochemical aspects of disinfection of water by means of ultrasound and ozone. Water Res. 10:677-684.
-
- Elovitz, M. S., U. von Gunten, and H. P. Kaiser. 2000. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties. Ozone Sci. Eng. 22:123-150.
-
- Facile, N., B. Barbeau, M. Prevost, and B. Koudjonou. 2000. Evaluating bacterial aerobic spores as a surrogate for Giardia and Cryptosporidium inactivation by ozone. Water Res. 34:3238-3246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

