Virus-contaminated oysters: a three-month monitoring of oysters imported to Switzerland
- PMID: 12676712
- PMCID: PMC154765
- DOI: 10.1128/AEM.69.4.2292-2297.2003
Virus-contaminated oysters: a three-month monitoring of oysters imported to Switzerland
Abstract
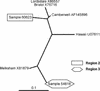
Molluscan shellfish are known to be carriers of viral and bacterial pathogens. The consumption of raw oysters has been repeatedly linked to outbreaks of viral gastroenteritis and hepatitis A. Switzerland imports over 300 tons of oysters per year, 95% of which originate in France. To assess the level of viral contamination, a 3-month monitoring study was conducted. Therefore, the sensitivities of several previously described methods for virus concentration were compared, and one protocol was finally chosen by using dissected digestive tissues. Eighty-seven samples consisting of five oysters each were analyzed for Norwalk-like viruses (NLVs), enteroviruses, and hepatitis A viruses from November 2001 to February 2002. The oysters were exported by 31 French, three Dutch, and two Irish suppliers. Eight oyster samples from six French suppliers were positive for NLVs, and four samples from four French suppliers were positive for enteroviruses; two of the latter samples were positive for both viral agents. No hepatitis A viruses were detected. The sequences of NLV and enterovirus amplicons showed a great variety of strains, especially for the NLVs (strains similar to Bristol, Hawaii, Mexico, and Melksham agent). The data obtained indicated that imported oysters might be a source of NLV infection in Switzerland. However, further studies are needed to determine the quantitative significance of the risk factor within the overall epidemiology of NLVs.
Figures


References
-
- Abeyta, C. 1983. Comparison of iron milk and official AOAC methods for enumeration of Clostridium perfringens from fresh seafood. J. Assoc. Off. Anal. Chem. 66:1175-1177. - PubMed
-
- Ando, T., S. S. Monroe, J. S. Noel., and R. I. Glass. 1997. A one-tube method of reverse transcription-PCR to efficiently amplify a 3-kilobase region from the RNA polymerase gene to the poly(A) tail of small round-structured viruses (Norwalk-like viruses). J. Clin. Microbiol. 35:570-577. - PMC - PubMed
-
- Atmar, R. L., F. H. Neil, C. M. Woodley, R. Manger, G. S. Fout, W. Burkhardt, L. Leja, E. R. McGovern, F. Le Guyader, T. G. Metcalf, and M. K. Estes. 1996. Collaborative evaluation of a method for the detection of Norwalk virus in shellfish tissues by PCR. Appl. Environ. Microbiol. 62:254-258. - PMC - PubMed
-
- Beuret, C., D. Kohler, and T. Lüthi. 2000. “Norwalk-like virus sequences” detected by reverse transcription-polymerase chain reaction in mineral waters imported into or bottled in Switzerland. J. Food Prot. 63:1576-1582. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

