Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa
- PMID: 12676715
- PMCID: PMC154819
- DOI: 10.1128/AEM.69.4.2313-2320.2003
Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa
Abstract
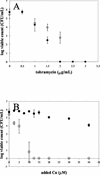
A study was undertaken to examine the effects of the heavy metals copper, lead, and zinc on biofilm and planktonic Pseudomonas aeruginosa. A rotating-disk biofilm reactor was used to generate biofilm and free-swimming cultures to test their relative levels of resistance to heavy metals. It was determined that biofilms were anywhere from 2 to 600 times more resistant to heavy metal stress than free-swimming cells. When planktonic cells at different stages of growth were examined, it was found that logarithmically growing cells were more resistant to copper and lead stress than stationary-phase cells. However, biofilms were observed to be more resistant to heavy metals than either stationary-phase or logarithmically growing planktonic cells. Microscopy was used to evaluate the effect of copper stress on a mature P. aeruginosa biofilm. The exterior of the biofilm was preferentially killed after exposure to elevated concentrations of copper, and the majority of living cells were near the substratum. A potential explanation for this is that the extracellular polymeric substances that encase a biofilm may be responsible for protecting cells from heavy metal stress by binding the heavy metals and retarding their diffusion within the biofilm.
Figures





References
-
- Breault, R. F., J. A. Colman, G. R. Aiken, and D. McKnight. 1996. Copper speciation and binding by organic matter in copper-contaminated streamwater. Environ. Sci. Technol. 30:3477-3486.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

