Comparison of two strategies for administering nevirapine to prevent perinatal HIV transmission in high-prevalence, resource-poor settings
- PMID: 12679702
- PMCID: PMC2745994
- DOI: 10.1097/00126334-200304150-00007
Comparison of two strategies for administering nevirapine to prevent perinatal HIV transmission in high-prevalence, resource-poor settings
Abstract
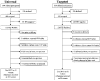
Universal nevirapine (NVP) therapy (provision of the drug without HIV testing) has been suggested as potentially superior to targeted NVP therapy (provision of the drug to seropositive patients identified through voluntary HIV counseling and testing [VCT]) for perinatal HIV prevention in low-resource, high-prevalence settings. The authors postulated that uptake (the proportion of women who accept the strategy when offered) may be higher for universal therapy, since it does not require a woman to learn her serostatus; they further postulated that adherence (the proportion of women who actually ingest the NVP tablet at labor onset) may be higher for targeted therapy, since knowledge of serostatus could motivate better adherence. Two clinics in Lusaka, Zambia were assigned to provide either the targeted or universal strategy. Halfway through the study period, the approach offered at each clinic was crossed over. Adherence was assessed by liquid chromatographic assay for NVP of cord blood. Regarding uptake, 1524 pregnant women were offered participation, and 1025 (67%) accepted. Of 694 women offered enrollment in the universal strategy, 496 (71%) accepted; of 830 women offered enrollment in the targeted strategy, 529 (64%) accepted (p <.01). Uptake was similar at both clinics for the universal strategy: 250 of 339 (74%) at clinic A and 246 of 355 (69%) at clinic B (p =.2), but differed significantly between clinics for the targeted strategy: 229 of 316 (72%) at clinic A and 300 of 514 (58%) at clinic B (RR, 1.51; 95% CI, 1.23, 1.86). Increased uptake correlated with having been offered the universal rather than the targeted strategy (AOR, 1.5; 95% CI, 1.1, 2.1), attendance at clinic A (AOR, 1.4; 95% CI, 1.01, 2.0), and maternal report of a prior fetal or infant death (AOR, 1.6; 95% CI, 1.1, 2.5). Regarding adherence, in the universal strategy, 40 of 103 women (39%) were nonadherent compared with 25 of 98 women (26%) in the targeted strategy (RR, 1.5; 95% CI, 1.004, 2.3). Failure to adhere correlated with participation in the universal strategy (AOR, 2.0; 95% CI, 1.04, 4.2) and illiteracy (AOR, 2.6; 95% CI, 1.2, 5.3). In high-prevalence settings with adequate VCT services, uptake of NVP using the universal or targeted approach appears comparable. However, the universal strategy may result in better uptake in clinics with less well-functioning VCT services (as with clinic B). Adherence to the single-dose NVP intervention was lower among women who did not learn their HIV status. Programs that seek to save the greatest possible number of infants from perinatal HIV acquisition should consider a combination approach, in which women who desire HIV testing can access NVP through a targeted strategy, and women who do not desire testing can access NVP through a universal strategy.
Figures
References
-
- UNAIDS . Report on the global HIV/AIDS epidemic: July 2002. UNAIDS; Geneva: 2002.
-
- Dabis F, Ekpini E. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359:2097–2104. - PubMed
-
- Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. - PubMed
-
- Fowler MG, Mwatha A, Guay L, et al. Effect of nevirapine (NVP) for perinatal HIV prevention appears greatest among women with most advanced disease: subgroup analyses of HIVNET 012 [abstract 120]. Presented at the 9th Conference on Retroviruses and Opportunistic Infections; Seattle. February 24–28, 2002.
-
- Marseille E, Kahn J, Mmiro F, et al. Cost effectiveness of single-dose nevirapine regimen for mothers and babies to decrease vertical HIV-1 transmission in sub-Saharan Africa. Lancet. 1999;354:803–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

