Hormonal regulation of dipeptide transporter (PepT1) in Caco-2 cells with normal and anoxia/reoxygenation management
- PMID: 12679938
- PMCID: PMC4611455
- DOI: 10.3748/wjg.v9.i4.808
Hormonal regulation of dipeptide transporter (PepT1) in Caco-2 cells with normal and anoxia/reoxygenation management
Abstract
Aim: To determine the regulation effects of recombinant human growth hormone (rhGH) on dipeptide transporter(PepT1) in Caco-2 cells with normal culture and anoxia/reoxygenation injury.
Methods: A human intestinal cell monolayer (Caco-2) was used as the in vitro model of human small intestine and cephalexin as the model substrate for dipeptide transporter (PepT1). Caco-2 cells grown on Transwell membrane filters were preincubated in the presence of rhGH in the culture medium for 4 d, serum was withdrawn from monolayers for 24 h before each experiment. The transport experiments of cephalexin across apical membromes were then conducted; Caco-2 cells grown on multiple well dishes (24 pore) with normal culture or anoxia/reoxygenation injury were preincubated with rhGH as above and uptake of cephalexin was then measured.
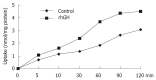
Results: The transport and uptake of cephelaxin across apical membranes of Caco-2 cells after preincubation with rhGH were significantly increased compared with controls (P=0.045, 0.0223). Also, addition of rhGH at physiological concentration (34 nM) to incubation medium greatly stimulates cephalexin uptake by anoxia/reoxygenation injuried Caco-2 cells (P=0.0116), while the biological functions of PepT1 in injured Caco-2 cells without rhGH were markedly downregulated. Northern blot analysis showed that the level of PepT1 mRNA of rhGH-treated injured Caco-2 cells was greatly increased compared to controls.
Conclusion: The present results of rhGH stimulating the uptake and transport of cephalexin indicated that rhGH greatly upregulates the physiological effects of dipeptide transporters of Caco-2 cells. The alteration in the gene expression may be a mechanism of regulation of PepT1. In addition, Caco-2 cells take up cephalexin by the Proton-dependent dipeptide transporters that closely resembles the transporters present in the intestine. Caco-2 cells represent an ideal cellular model for future studies of the dipeptide transporter.
Figures




Similar articles
-
Changes of biological functions of dipeptide transporter (PepT1) and hormonal regulation in severe scald rats.World J Gastroenterol. 2003 Dec;9(12):2782-5. doi: 10.3748/wjg.v9.i12.2782. World J Gastroenterol. 2003. PMID: 14669333 Free PMC article.
-
Uptake of the cephalosporin, cephalexin, by a dipeptide transport carrier in the human intestinal cell line, Caco-2.Biochim Biophys Acta. 1990 Sep 7;1027(3):211-7. doi: 10.1016/0005-2736(90)90309-c. Biochim Biophys Acta. 1990. PMID: 2397233
-
PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine.J Clin Invest. 2001 Nov;108(10):1483-94. doi: 10.1172/JCI13219. J Clin Invest. 2001. PMID: 11714740 Free PMC article.
-
Di/tri-peptide transporters as drug delivery targets: regulation of transport under physiological and patho-physiological conditions.Curr Drug Targets. 2003 Jul;4(5):373-88. doi: 10.2174/1389450033491028. Curr Drug Targets. 2003. PMID: 12816347 Review.
-
Membrane transporters.Eur J Pharm Sci. 2000 Oct;11 Suppl 2:S41-50. doi: 10.1016/s0928-0987(00)00163-9. Eur J Pharm Sci. 2000. PMID: 11033426 Review.
Cited by
-
Changes of biological functions of dipeptide transporter (PepT1) and hormonal regulation in severe scald rats.World J Gastroenterol. 2003 Dec;9(12):2782-5. doi: 10.3748/wjg.v9.i12.2782. World J Gastroenterol. 2003. PMID: 14669333 Free PMC article.
-
Regulation profile of the intestinal peptide transporter 1 (PepT1).Drug Des Devel Ther. 2017 Dec 8;11:3511-3517. doi: 10.2147/DDDT.S151725. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29263649 Free PMC article. Review.
References
-
- Vincent ML, Russell RM, Sasak V. Folic acid uptake characteristics of a human colon carcinoma cell line, Caco-2. A newly-described cellular model for small intestinal epithelium. Hum Nutr Clin Nutr. 1985;39:355–360. - PubMed
-
- Mohrmann I, Mohrmann M, Biber J, Murer H. Sodium-dependent transport of Pi by an established intestinal epithelial cell line (CaCo-2) Am J Physiol. 1986;250:G323–G330. - PubMed
-
- Blais A, Bissonnette P, Berteloot A. Common characteristics for Na+-dependent sugar transport in Caco-2 cells and human fetal colon. J Membr Biol. 1987;99:113–125. - PubMed
-
- Rousset M, Laburthe M, Pinto M, Chevalier G, Rouyer-Fessard C, Dussaulx E, Trugnan G, Boige N, Brun JL, Zweibaum A. Enterocytic differentiation and glucose utilization in the human colon tumor cell line Caco-2: modulation by forskolin. J Cell Physiol. 1985;123:377–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

