A redox-sensitive loop regulates plasminogen activator inhibitor type 2 (PAI-2) polymerization
- PMID: 12682008
- PMCID: PMC154470
- DOI: 10.1093/emboj/cdg178
A redox-sensitive loop regulates plasminogen activator inhibitor type 2 (PAI-2) polymerization
Abstract
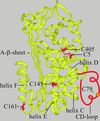
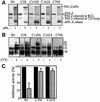
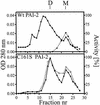
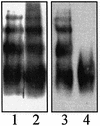
Plasminogen activator inhibitor type 2 (PAI-2) is the only wild-type serpin that polymerizes spontaneously under physiological conditions. We show that PAI-2 loses its ability to polymerize following reduction of thiol groups, suggesting that an intramolecular disulfide bond is essential for the polymerization. A novel disulfide bond was identified between C79 (in the CD-loop) and C161 (at the bottom of helix F). Substitution mutants in which this disulfide bond was broken did not polymerize. Reactive center loop peptide insertion experiments and binding of bis-ANS to hydrophobic cavities indicate that the C79-C161 disulfide bond stabilizes PAI-2 in a polymerogenic conformation with an open A-beta-sheet. Elimination of this disulfide bond causes A-beta-sheet closure and abrogates the polymerization. The finding that cytosolic PAI-2 is mostly monomeric, whereas PAI-2 in the secretory pathway is prone to polymerize, suggests that the redox status of the cell could regulate PAI-2 polymerization. Taken together, our data suggest that the CD-loop functions as a redox-sensitive switch that converts PAI-2 between an active stable monomeric and a polymerogenic conformation, which is prone to form inactive polymers.
Figures


Similar articles
-
Structural bases of the redox-dependent conformational switch in the serpin PAI-2.J Mol Biol. 2004 Dec 10;344(5):1359-68. doi: 10.1016/j.jmb.2004.10.010. J Mol Biol. 2004. PMID: 15561148
-
VLHL plasminogen activator inhibitor spontaneously reactivates from the latent to active form.Int J Mol Med. 2009 Jan;23(1):57-63. Int J Mol Med. 2009. PMID: 19082507
-
Plasminogen activator inhibitor-1 is locked in active conformation and polymerizes upon binding ligands neutralizing its activity.Int J Mol Med. 2006 Mar;17(3):437-47. Int J Mol Med. 2006. PMID: 16465390
-
The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2.FEBS J. 2005 Oct;272(19):4858-67. doi: 10.1111/j.1742-4658.2005.04879.x. FEBS J. 2005. PMID: 16176260 Review.
-
The structural basis for the pathophysiological relevance of PAI-I in cardiovascular diseases and the development of potential PAI-I inhibitors.Thromb Haemost. 2004 Mar;91(3):425-37. doi: 10.1160/TH03-12-0764. Thromb Haemost. 2004. PMID: 14983217 Review.
Cited by
-
Bomapin is a redox-sensitive nuclear serpin that affects responsiveness of myeloid progenitor cells to growth environment.BMC Cell Biol. 2010 Apr 30;11:30. doi: 10.1186/1471-2121-11-30. BMC Cell Biol. 2010. PMID: 20433722 Free PMC article.
-
A Novel Role for Plasminogen Activator Inhibitor Type-2 as a Hypochlorite-Resistant Serine Protease Inhibitor and Holdase Chaperone.Cells. 2022 Mar 29;11(7):1152. doi: 10.3390/cells11071152. Cells. 2022. PMID: 35406715 Free PMC article.
-
The myxoid/round cell liposarcoma fusion oncogene FUS-DDIT3 and the normal DDIT3 induce a liposarcoma phenotype in transfected human fibrosarcoma cells.Am J Pathol. 2006 May;168(5):1642-53. doi: 10.2353/ajpath.2006.050872. Am J Pathol. 2006. PMID: 16651630 Free PMC article.
-
Probing the local conformational change of alpha1-antitrypsin.Protein Sci. 2007 Sep;16(9):1842-50. doi: 10.1110/ps.072911607. Epub 2007 Jul 27. Protein Sci. 2007. PMID: 17660256 Free PMC article.
-
SOD1-associated ALS: a promising system for elucidating the origin of protein-misfolding disease.HFSP J. 2008 Dec;2(6):354-64. doi: 10.2976/1.2995726. Epub 2008 Oct 14. HFSP J. 2008. PMID: 19436494 Free PMC article.
References
-
- Bachmann F. (1995) The enigma PAI-2. Gene expression, evolutionary and functional aspects. Thromb. Haemost., 74, 172–179. - PubMed
-
- Baumann U., Huber,R., Bode,W., Grosse,D., Lesjak,M. and Laurell,C.B. (1991) Crystal structure of cleaved human α1-antichymotrypsin at 2.7 Å resolution and its comparison with other serpins. J. Mol. Biol., 218, 595–606. - PubMed
-
- Björk I., Ylinenjarvi,K., Olson,S.T. and Bock,P.E. (1992) Conversion of antithrombin from an inhibitor of thrombin to a substrate with reduced heparin affinity and enhanced conformational stability by binding of a tetradecapeptide corresponding to the P1 to P14 region of the putative reactive bond loop of the inhibitor. J. Biol. Chem., 267, 1976–1982. - PubMed
-
- Björquist P., Ehnebom,J., Inghardt,T., Hansson,L., Lindberg,M., Linschoten,M., Stroemqvist,M. and Deinum,J. (1998) Identification of the binding site for a low-molecular-weight inhibitor of plasminogen activator inhibitor type 1 by site-directed mutagenesis. Biochemistry, 37, 1227–1234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

