Structure of the GCM domain-DNA complex: a DNA-binding domain with a novel fold and mode of target site recognition
- PMID: 12682016
- PMCID: PMC154474
- DOI: 10.1093/emboj/cdg182
Structure of the GCM domain-DNA complex: a DNA-binding domain with a novel fold and mode of target site recognition
Abstract
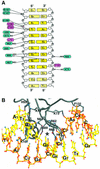
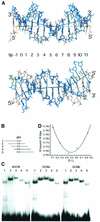
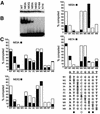
Glia cell missing (GCM) transcription factors form a small family of transcriptional regulators in metazoans. The prototypical Drosophila GCM protein directs the differentiation of neuron precursor cells into glia cells, whereas mammalian GCM proteins are involved in placenta and parathyroid development. GCM proteins share a highly conserved 150 amino acid residue region responsible for DNA binding, known as the GCM domain. Here we present the crystal structure of the GCM domain from murine GCMa bound to its octameric DNA target site at 2.85 A resolution. The GCM domain exhibits a novel fold consisting of two domains tethered together by one of two structural Zn ions. We observe the novel use of a beta-sheet in DNA recognition, whereby a five- stranded beta-sheet protrudes into the major groove perpendicular to the DNA axis. The structure combined with mutational analysis of the target site and of DNA-contacting residues provides insight into DNA recognition by this new type of Zn-containing DNA-binding domain.
Figures


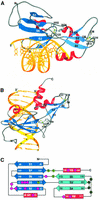

Similar articles
-
The GCM domain is a Zn-coordinating DNA-binding domain.FEBS Lett. 2002 Sep 25;528(1-3):95-100. doi: 10.1016/s0014-5793(02)03257-x. FEBS Lett. 2002. PMID: 12297286
-
Structural requirements for DNA binding of GCM proteins.Nucleic Acids Res. 1998 May 15;26(10):2337-43. doi: 10.1093/nar/26.10.2337. Nucleic Acids Res. 1998. PMID: 9580683 Free PMC article.
-
Conservation and variation of structure and function in a newly identified GCM homolog from chicken.J Mol Biol. 2004 Feb 13;336(2):441-51. doi: 10.1016/j.jmb.2003.12.029. J Mol Biol. 2004. PMID: 14757056
-
Impacts of a new transcription factor family: mammalian GCM proteins in health and disease.J Cell Biol. 2004 Sep 13;166(6):765-8. doi: 10.1083/jcb.200406097. Epub 2004 Sep 7. J Cell Biol. 2004. PMID: 15353544 Free PMC article. Review.
-
Chronicles of a switch hunt: gcm genes in development.Trends Genet. 2001 May;17(5):286-90. doi: 10.1016/s0168-9525(01)02275-2. Trends Genet. 2001. PMID: 11335039 Review.
Cited by
-
Plant-Produced Viral Nanoparticles as a Functionalized Catalytic Support for Metabolic Engineering.Plants (Basel). 2024 Feb 11;13(4):503. doi: 10.3390/plants13040503. Plants (Basel). 2024. PMID: 38498408 Free PMC article.
-
Global Expressions Landscape of NAC Transcription Factor Family and Their Responses to Abiotic Stresses in Citrullus lanatus.Sci Rep. 2016 Aug 5;6:30574. doi: 10.1038/srep30574. Sci Rep. 2016. PMID: 27491393 Free PMC article.
-
A novel mutation in the GCM2 gene causing severe congenital isolated hypoparathyroidism.J Pediatr Endocrinol Metab. 2012;25(7-8):741-6. doi: 10.1515/jpem-2012-0080. J Pediatr Endocrinol Metab. 2012. PMID: 23155703 Free PMC article.
-
Structure, function and networks of transcription factors involved in abiotic stress responses.Int J Mol Sci. 2013 Mar 13;14(3):5842-78. doi: 10.3390/ijms14035842. Int J Mol Sci. 2013. PMID: 23485989 Free PMC article.
-
Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors.EMBO Rep. 2004 Mar;5(3):297-303. doi: 10.1038/sj.embor.7400093. EMBO Rep. 2004. PMID: 15083810 Free PMC article.
References
-
- Akiyama-Oda Y., Hotta,Y., Tsukita,S. and Oda,H. (2000) Mechanism of glia-neuron cell-fate switch in the Drosophila thoracic neuroblast 6-4 lineage. Development, 127, 3513–3522. - PubMed
-
- Anson-Cartwright L., Dawson,K., Holmyard,D., Fisher,S.J., Lazzarini,R.A. and Cross,J.C. (2000) The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet., 25, 311–314. - PubMed
-
- Berg J.M. and Shi,Y. (1996) The galvanization of biology: a growing appreciation for the roles of zinc. Science, 271, 1081–1085. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

