GDNF promotes tubulogenesis of GFRalpha1-expressing MDCK cells by Src-mediated phosphorylation of Met receptor tyrosine kinase
- PMID: 12682085
- PMCID: PMC2172872
- DOI: 10.1083/jcb.200212174
GDNF promotes tubulogenesis of GFRalpha1-expressing MDCK cells by Src-mediated phosphorylation of Met receptor tyrosine kinase
Abstract
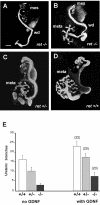
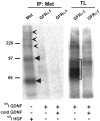
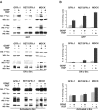
Glial cell line-derived neurotrophic factor (GDNF) and hepatocyte growth factor (HGF) are multifunctional signaling molecules in embryogenesis. HGF binds to and activates Met receptor tyrosine kinase. The signaling receptor complex for GDNF typically includes both GDNF family receptor alpha1 (GFRalpha1) and Ret receptor tyrosine kinase. GDNF can also signal independently of Ret via GFRalpha1, although the mechanism has remained unclear. We now show that GDNF partially restores ureteric branching morphogenesis in ret-deficient mice with severe renal hypodysplasia. The mechanism of Ret-independent effect of GDNF was therefore studied by the MDCK cell model. In MDCK cells expressing GFRalpha1 but no Ret, GDNF stimulates branching but not chemotactic migration, whereas both branching and chemotaxis are promoted by GDNF in the cells coexpressing Ret and GFRalpha1, mimicking HGF/Met responses in wild-type MDCK cells. Indeed, GDNF induces Met phosphorylation in several ret-deficient/GFRalpha1-positive and GFRalpha1/Ret-coexpressing cell lines. However, GDNF does not immunoprecipite Met, making a direct interaction between GDNF and Met highly improbable. Met activation is mediated by Src family kinases. The GDNF-induced branching of MDCK cells requires Src activation, whereas the HGF-induced branching does not. Our data show a mechanism for the GDNF-induced branching morphogenesis in non-Ret signaling.
Figures




Similar articles
-
GDNF triggers a novel ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor alpha1.FEBS Lett. 1999 Dec 10;463(1-2):63-6. doi: 10.1016/s0014-5793(99)01590-2. FEBS Lett. 1999. PMID: 10601639
-
Internalization of glial cell-derived neurotrophic factor receptor GFR alpha 1 in the absence of the ret tyrosine kinase coreceptor.Cell Mol Neurobiol. 2003 Feb;23(1):43-55. doi: 10.1023/a:1022593001155. Cell Mol Neurobiol. 2003. PMID: 12701883 Free PMC article.
-
Crosstalk between Jagged1 and GDNF/Ret/GFRalpha1 signalling regulates ureteric budding and branching.Mech Dev. 2005 Jun;122(6):765-80. doi: 10.1016/j.mod.2005.03.006. Mech Dev. 2005. PMID: 15905075
-
Novel functions and signalling pathways for GDNF.J Cell Sci. 2003 Oct 1;116(Pt 19):3855-62. doi: 10.1242/jcs.00786. J Cell Sci. 2003. PMID: 12953054 Review.
-
GDNF recruits the signaling crew into lipid rafts.Trends Neurosci. 2001 Aug;24(8):427-9. doi: 10.1016/s0166-2236(00)01864-6. Trends Neurosci. 2001. PMID: 11476867 Review.
Cited by
-
RET is a potential tumor suppressor gene in colorectal cancer.Oncogene. 2013 Apr 18;32(16):2037-47. doi: 10.1038/onc.2012.225. Epub 2012 Jul 2. Oncogene. 2013. PMID: 22751117 Free PMC article.
-
A potential role of progestin-induced laminin-5/α6-integrin signaling in the formation of side branches in the mammary gland.Endocrinology. 2012 Oct;153(10):4990-5001. doi: 10.1210/en.2012-1518. Epub 2012 Aug 21. Endocrinology. 2012. PMID: 22910029 Free PMC article.
-
Fas ligand enhances malignant behavior of tumor cells through interaction with Met, hepatocyte growth factor receptor, in lipid rafts.J Biol Chem. 2012 Jun 8;287(24):20664-73. doi: 10.1074/jbc.M111.326058. Epub 2012 Apr 25. J Biol Chem. 2012. PMID: 22535954 Free PMC article.
-
A holey pursuit: lumen formation in the developing kidney.Pediatr Nephrol. 2017 Jan;32(1):7-20. doi: 10.1007/s00467-016-3326-4. Epub 2016 Feb 22. Pediatr Nephrol. 2017. PMID: 26902755 Free PMC article. Review.
-
RET-independent signaling by GDNF ligands and GFRα receptors.Cell Tissue Res. 2020 Oct;382(1):71-82. doi: 10.1007/s00441-020-03261-2. Epub 2020 Jul 31. Cell Tissue Res. 2020. PMID: 32737575 Free PMC article. Review.
References
-
- Abram, C.L., and S.A. Courtneidge. 2000. Src family tyrosine kinases and growth factor signaling. Exp. Cell Res. 254:1–13. - PubMed
-
- Airaksinen, M.S., A. Titievsky, and M. Saarma. 1999. GDNF family neurotrophic factor signaling: four masters, one servant? Mol. Cell. Neurosci. 13:313–325. - PubMed
-
- Baloh, R.H., H. Enomoto, E.M. Johnson, Jr., and J. Milbrandt. 2000. The GDNF family ligands and receptors—implications for neural development. Curr. Opin. Neurobiol. 10:103–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

